
Long-COVID syndrome in hospitalized patients after 2 years of follow-up
Highlight box
Key findings
• COVID-19; SARS-CoV-2; post-COVID syndrome; antiviral therapy.
What is known and what is new?
• Post-Covid syndrome affects about 20–40% of infected subjects, but clinical data at longer time of follow-up are not currently reported.
• At 2 years of follow-up, about 40% of enrolled subjects was affected by the post-COVID syndrome; only 8.5% had severe limitations in everyday life.
What is the implication, and what should change now?
• Long-COVID is a clinical challenge also after 2 years of follow-up. Early intervention and treatment of viral infection should be the optimal approach.
Introduction
Coronaviruses are a group of RNA viruses currently classified in the order Nidovirales. All the coronaviruses responsible for respiratory syndrome are β-coronaviruses (1,2). The coronavirus disease 2019 (COVID-19) due to the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged as global pandemic as reported by the World Health Organization (WHO) on the 11th March 2020 and quickly become a global health emergency (3). Main clinical manifestations in the acute phase of viral infections were respiratory, cardiovascular and multi-organ involvement (4); however, in the last two years, several studies highlighted persistence of multiorgan dysfunction after hospital discharge in patients with acute COVID-19 (5). Post-viral systemic sequelae were observed also after other coronavirus diseases such as the Middle East respiratory syndrome coronavirus (MERS) and the severe acute respiratory syndrome coronavirus (SARS) (6), and acute COVID-19 (7-10). As reported in these studies, the most frequent symptoms were: fever, fatigue, breathlessness, headaches, cough, cognitive blunting (“brain fog”), anxiety and depression, muscle pains, arthralgias, paraesthesias, etc. (7,11). Risk factors, severity grade of presentation and duration of the post-COVID-19 syndrome (PCS) are currently subjects for discussion; in some studies the symptoms’ severity and duration were related to the patients’ clinical characteristics or hospitalization course: admission in the intensive care unit (ICU), need of mechanical ventilation, comorbidities, older age, etc. (12), but with variables findings in different populations (13); this may depend by the higher heterogeneity among the various studies involving subjects with different illness severity and follow-up period. Because of this, a novel definition of PCS according to the time of follow-up and the duration of symptoms was recently proposed: acute-PCS, within 12 weeks after hospital discharge, long-PCS between 12–24 weeks and persistent-PCS after 24 weeks (14). This approach maybe interesting because could allow to distinguish the real PCS from non-specific symptoms mainly related to the hospitalization and more frequent in the first time of follow-up; the persistence of PCS after 12–24 weeks, on the other hand, can be more correlated with the immunological consequence due to the “viral trigger” of SARS-CoV-2 infection (15).
The main purpose of this study was the analysis of prevalence and the risk factors of PCS in a cohort of hospitalized patients with a long-term follow-up (2 years) after hospital discharge. We present this article in accordance with the STROBE reporting checklist (available at https://jphe.amegroups.com/article/view/10.21037/jphe-22-66/rc).
Methods
Study design and definitions
We conducted a prospective study including all patients with diagnosis of COVID-19 hospitalized from 10th March 2020 to 15th January 2021 at our center of infectious diseases at “Saint Andrea Hospital”, Vercelli, Italy, and followed in our “post-COVID ambulatory” for at least 2 years after discharge. At the follow-up visit a structured interview was done to register global health status and clinical scores; demographic, clinical, laboratory and radiological data were obtained. All patients were included in this study after acceptance of study protocol and informed consent. The study protocol was approved by the local Ethics Committee: ‘Comitato Etico Interaziendale ASL VC’ (4/8/2020; Protocol number: 0026301). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all individual participants included in this study.
Study endpoints
The primary outcome of this study was the assessment of prevalence and severity of PCS in the enrolled patients after 6 months of follow-up period. The level of functional status and the severity of PCS were reported using the “post-COVID-19 functional status scale (PCFS)” defining the presence of significant PCS for values >2. Grade 0–1 was referred to the absence of limitations or presence of negligible limitations; grade 2 is related to mild limitations that do not affect the everyday life; grade 3–4 were referred to significant limitations in everyday life with important functional limitations (16). The Fatigue Assessment Scale (FAS) was used to quantify the level of fatigue reported in the follow-up: the total score ranges from 10 to 50 points; 0–21: no fatigue; 22–34: mild/moderate fatigue; ≥35: severe fatigue (17). The Hospital Anxiety and Depression Scale (HADS) was used to identify patients with significant anxiety or depression disorders (cut-off ≥8) (18).
Statistical analysis
In descriptive statistics continuous variables were summarized as median [interquartile range (IQR): 25th to 75th percentiles]. Categorical variables were described as frequency and percentage. All data were assessed for normality using a Shapiro-Wilk test and categorical data were compared using a Mann Whitney or Kruskal-Wallis statistical test. To investigate continuous data, a Spearman Rank correlation was utilized. The association was calculated using the χ2-test. Multivariate logistic regression analysis with stepwise forward selection was performed to evaluate the factors associated with the PCS presence, with P values of less than 0.05 as the criteria for model inclusion. All P values were two-tailed. P<0.05 was considered statistically significant. Statistical analyses were conducted by using SPSS software package ver. 28.0 (Chicago, IL, USA).
Results
Patients’ selection and baseline characteristics
A total of 361 patients were discharged after hospital admission due to COVID-19 in the study period. Excluded subjects were 55 for the following reasons: 12 died after hospital discharge, 19 were unavailable to follow-up and 24 were lost at follow-up. Finally, 306 patients were included in the analysis (Figure 1). In the Table 1 were described the baseline characteristics of the study population. Median age was 66.5 years; male patients were 225 (73.5%); obesity was observed in 44 subjects (14.4%); the most frequent comorbidities were cardiovascular diseases (27.1%), diabetes (49.3%), neurological diseases (16.7%) and chronic obstructive pulmonary diseases (COPD) (11.8%). Median time from the onset of symptoms and the hospital admission was 11.5 days; the median time of follow-up was 25.5 months. 178 subjects received continuous positive airway pressure (CPAP)/non-invasive ventilation (NIV) (58.2%), 81 needed of ICU admission (26.5%); median time of hospitalization was 14.5 days. Sepsis was observed in 54 (17.6%), nosocomial infections in 112 (36.6%); 169 were discharged at home (55.2%), 137 in long-term care facilities (44.8%). 14 patients (4.6%) were further re-hospitalized due to other clinical conditions.
Table 1
| Characteristics | Values (N=306 patients) |
|---|---|
| Demographics | |
| Age (years), median (IQR) | 66.5 (58.5–78.5) |
| Male, n (%) | 225 (73.5) |
| BMI (kg/m2), n (%) | |
| <25 | 71 (23.2) |
| 25–30 | 191 (62.4) |
| >30 | 44 (14.4) |
| Ethnicity, n (%) | |
| Italian | 281 (91.8) |
| East Europe | 8 (2.6) |
| Asian | 4 (1.3) |
| African | 13 (4.2) |
| Smoking status, n (%) | |
| Current | 81 (26.5) |
| Former | 78 (25.5) |
| Never | 147 (48.0) |
| Working status, n (%) | |
| Employed | 71 (23.2) |
| Retired | 181 (59.2) |
| Unemployed | 54 (17.6) |
| Comorbidities, n (%) | |
| Cardiovascular disease | 83 (27.1) |
| COPD | 36 (11.8) |
| Chronic kidney disease | 14 (4.6) |
| Diabetes | 151 (49.3) |
| Neurological chronic disease | 51 (16.7) |
| Psychiatric disease | 31 (10.1) |
| Neoplastic disease | 18 (5.9) |
| Immunological disease | 8 (2.6) |
| Chronic liver disease | 32 (10.5) |
| Days from the onset of symptoms to hospital admission, median (IQR) | 11.5 (8.4–16.8) |
| Time of follow-up (months), median (IQR) | 25.5 (21.0–30.5) |
| Outcomes and clinical features | |
| Days of hospitalization, median (IQR) | 14.5 (9.6–31.8) |
| Need of CPAP/NIV, n (%) | 178 (58.2) |
| Need of ICU admission, n (%) | 81 (26.5) |
| Sepsis, n (%) | 54 (17.6) |
| Nosocomial infections, n (%) | 112 (36.6) |
| Discharged at home, n (%) | 169 (55.2) |
| Discharged at long-term care facilities, n (%) | 137 (44.8) |
| Need of oxygen at home, n (%) | 227 (74.2) |
| Need of rehabilitation after discharge, n (%) | 183 (59.8) |
| Need of rehospitalization after discharge, n (%) | 14 (4.6) |
IQR, interquartile range; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; NIV, non-invasive ventilation; ICU, intensive care unit.
Clinical outcomes
In the Table 2 was described the overall follow-up evaluation in the study population; the PCS was diagnosed in 134 subjects (43.8%); according to the PCFS scale we observed 188 patients with score 0–1, 78 with score 2 and 40 with score 3–4. According to FAS we observed 189 subjects with no fatigue (61.8%), 91 (29.7%) with mild/moderate fatigue and 26 (8.5%) with severe fatigue.
Table 2
| Follow-up evaluation | Subcategory | N (%) (n=306) |
|---|---|---|
| Overall PCS | – | 134 (43.8) |
| Systemic symptoms | Fatigue | 117 (38.2) |
| Myalgias/arthralgias | 14 (4.6) | |
| Fever | 2 (0.7) | |
| Headache | 77 (25.2) | |
| Pneumological symptoms | Dyspnea/breathlessness | 59 (19.3) |
| Cough | 21 (6.9) | |
| Chest pain | 6 (2.0) | |
| Neurological symptoms | “Brain fog” | 91 (29.7) |
| Dizziness | 11 (3.6) | |
| Memory impairment | 64 (20.9) | |
| Anosmia | 44 (14.4) | |
| Ageusia/dysgeusia | 24 (7.8) | |
| Peripheral neuropathy | 23 (7.5) | |
| Cardiovascular symptoms | Arrhythmias | 49 (16.0) |
| Pericarditis/myocarditis | 9 (2.9) | |
| Myocardial infarction | 4 (1.3) | |
| Psychiatric symptoms | Sleeping disorders | 88 (28.8) |
| Post-traumatic stress disorder | 90 (29.4) | |
| Anxiety | 122 (39.9) | |
| Major depression | 31 (10.1) | |
| Psychosis | 4 (1.3) | |
| Behavior disorder | 2 (0.7) | |
| Other clinical conditions | Weight loss | 24 (7.8) |
| Hair loss | 31 (10.1) | |
| Diabetes | 40 (13.1) | |
| Hypertension | 36 (11.8) | |
| Psoriasis | 11 (3.6) | |
| Venous thromboembolism | 10 (3.3) | |
| Thyroid dysfunction | 17 (5.5) | |
| Radiological characteristics | Interstitial lung abnormalities | 38 (12.4) |
| Post-COVID-19 functional status scale | 0–1 | 188 (61.4) |
| 2 | 78 (25.5) | |
| 3–4 | 40 (13.1) | |
| Fatigue assessment scale | 10–21 (no fatigue) | 189 (61.8) |
| 22–34 (mild/moderate fatigue) | 91 (29.7) | |
| ≥35 (severe fatigue) | 26 (8.5) | |
| Working status | Jobless after hospital admission | 13 (4.2) |
| Reduced working ability | 19 (6.2) | |
| Retired after hospital admission | 11 (3.6) | |
| Late resumption of work (>6 months) | 6 (2.0) | |
| Normal resumption of work | 22 (7.2) |
PCS, post-COVID syndrome; COVID-19, coronavirus disease 2019.
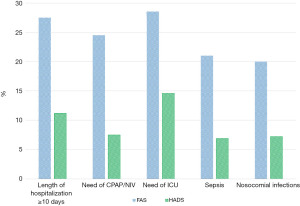
The follow-up evaluation of clinical condition revealed that the most common symptoms were: fatigue (38.2%), headache (25.2%), breathlessness (19.3%), “brain fog” (29.7%), sleeping disorders (28.8%), anxiety (39.9%), stress disorders (29.4%), memory impairment (20.9%). Interstitial lung abnormalities were present in 38 patients (12.4%). In baseline working status of enrolled patients, we reported that 71 of them (23.2%) were employed at the time of hospital admission. At the follow-up visit 22 patients resumed the work without limitations (31%), 13 were jobless after hospital admission (18%), 19 had a reduced working ability (27%), 11 were retired after hospital discharge (16%) and 6 were late resumption of work (8%) (Figure 2). According to FAS and HADS score we reported in Figure 3 the different values observed in patients with hospitalization time longer than 10 days, need of CPAP/NIV, need of ICU, presence of sepsis and nosocomial infections.
Analysis of risk factors for the presence of PCS in the study population
Descriptive clinical variables were assessed in the univariate logistic regression: age, sex, BMI, ethnicity, smoking status, comorbidities, days from the onset of symptoms to hospital admission, length of hospitalization, presence of sepsis, nosocomial infection, need of ICU, need of CPAP/NIV. All variables with P<0.20 were evaluated in the multivariate logistic regression.
After multivariate adjustment the following parameters resulted independent predictors of PCS: ICU admission (OR =3.950; 95% CI: 2.466–8.112; P=0.002), length of hospitalization (OR =1.855; 95% CI: 1.248–5.223; P=0.004) and nosocomial infections (OR =2.556; 95% CI: 1.443–5.292; P<0.001).
Discussion
After 2 years of follow-up, a significant proportion of patients discharged after hospital admission due to COVID-19 in the first wave of pandemic was still affected by PCS. In facts, 43.8% of enrolled subjects suffered of PCS after 2 years of follow-up; in our previous study we reported that PCS was observed in 71% of patients at the first follow-up visit (1 month) and 45% at the second visit (6 months) (19). These data confirmed the main findings observed in other studies, with some different due to patient’s enrollment, time of follow-up and PCS definition (20,21). However, the severity of different clinical symptoms was significantly lower at 2 years of follow-up as compared to 6 months and 1 year: only 13.1% of patients had a PCFS score >3, and 8.5% with severe fatigue assessment. Mild fatigue, “brain fog” and memory loss, anxiety and sleep disorders were finally the most prevalent observed symptoms. An interesting debatable point is the lack of agreement in the definition of PCS. Although several proposals were available, the most common description was the presence of COVID-19 related symptoms for more than three months after the diagnosis of SARS-CoV-2 infection or symptoms’ onset (22). Different proposals as “chronic COVID syndrome, long-COVID, post-COVID, post-acute COVID” were based on symptoms’ timing and their magnitude, with a relevant difference between PCS observed in hospitalized or non-hospitalized patients (14). In outpatients the diagnosis could be difficult due to the asymptomatic phase of infection or the lack of diagnosis by PCR. Furthermore, in the early phase of follow-up (within 6 months after discharge) most subjects suffered from an “acute-post-COVID syndrome”, with higher prevalence of signs and symptoms mainly related to hospitalization: weight loss, myalgias, polyneuropathies, dysphonia, trouble walking, sleep disorders and psychiatric symptoms such as anxiety, depression and post-traumatic stress disorder that were frequently related to prolonged isolation period and the “fear of death” experienced during the hospitalization. Other clinical manifestations as diabetes or hypertension were often consequent to the use of high dose of corticosteroids and CPAP/NIV. Although the frequency and duration of follow-up is currently not defined, a real “long-COVID or persistent-COVID” was referred to a longer time of follow-up (>1 year) and this condition was relevant both in clinical and non-clinical conditions in the overall limitation and worsening of everyday life. The pathophysiology was still unknown, involving a possible post-viral syndrome, especially for hearth (tachycardia, myocarditis, pericarditis), lung (dyspnea, cough, chest pain, breathlessness) and central nervous system (“brain fog”, anosmia, ageusia, psychosis) or prolonged inflammation syndrome determinant in “fatigue syndrome”, headache, autoimmune diseases, dizziness, fever, etc. In our study we observe that after 2 years of follow up fatigue (38.2%), breathlessness (19.3%), “brain fog” (29.7%), sleeping disorders (28.8%), post-traumatic stress disorder (29.4%) and anxiety (39.9%) were the most frequent clinical manifestations of PCS. Despite a severe limitation in everyday life was documented in only 8.5% of the patients, some of these symptoms had a negative impact on the quality of life mainly in non-clinical setting. Our analysis revealed that a condition of general and psychological distress due to viral infection, hospitalization and emotional consequences have a negative impact on the quality of life and related psychiatric symptoms such as anxiety, depression, and post-traumatic stress disorders; furthermore, the “brain fog” condition was mainly related to worsening in working ability, social relations and other activities. The working status at follow-up, surprisingly, revealed that only 7.2% of patients resumed the work after hospital discharge without reduced ability or need of rest period. This is a new data that we believe should focus the attention on this topic still little known and studied.
Our logistic regression showed that the ICU admission, nosocomial infection and the hospitalization time were the most important factor related to the PCS; interestingly, age and comorbidities were not predictive of PCS, that follows from the SARS-CoV-2 infection also in younger patients without other illness. Treatment of PCS was based on the symptoms management and rehabilitation (physical, psychological, and psychiatric) with a multidisciplinary approach including appropriate specialistic support. No specific pharmacology treatment is available for “brain fog” syndrome with cognitive impairment and more studies are needed to better understand the management of these conditions.
This study presents some strengths: follow-up time longer than most of previous studies, real-life intervention with clinical evaluation, blood test, radiographs and other relevant tests, analysis of both clinical and non-clinical aspects of PCS.
Main limitations of this study are: patients’ selection based on the hospitalization, with a 26.5% of ICU admission, could represent a population with greater incidence of PCS than non-hospitalized subjects; the evaluation period of the first follow-up at 2 years included patients in the first wave of pandemic, with consequent more aggressive disease and less effective treatment and management: for these reasons the prevalence of PCS could be higher than in other waves.
Conclusions
Although the long-term follow-up of PCS is relatively new medical condition, after 2 years a significant rate of patients suffers from this syndrome, even if a small part of them is characterized by a severe limitation in everyday life. Working/social condition of patients and the “brain fog” syndrome required more attention in further studies.
Acknowledgments
We would like to thank the study participants: we shared with them their sufferings, the isolation, the fear of death, the loss of affections and finally the hope of going home. It was an unforgettable experience, for better or for worse.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Public Health and Emergency for the series “Clinical and Non-clinical Consequences after COVID-19 Recovery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at: https://jphe.amegroups.com/article/view/10.21037/jphe-22-66/rc
Data Sharing Statement: Available at https://jphe.amegroups.com/article/view/10.21037/jphe-22-66/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jphe.amegroups.com/article/view/10.21037/jphe-22-66/coif). The series “Clinical and Non-clinical Consequences after COVID-19 Recovery” was commissioned by the editorial office without any funding or sponsorship. LB serves as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Public Health and Emergency from September 2020 to September 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the local Ethics Committee: ‘Comitato Etico Interaziendale ASL VC’ (4/8/2020; Protocol number: 0026301). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all individual participants included in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2019;17:181-92. [Crossref] [PubMed]
- Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol 2020;92:418-23. [Crossref] [PubMed]
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020;324:782-93. [Crossref] [PubMed]
- Tabary M, Khanmohammadi S, Araghi F, et al. Pathologic features of COVID-19: A concise review. Pathol Res Pract 2020;216:153097. [Crossref] [PubMed]
- Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ 2021;372: [Crossref] [PubMed]
- Ngai JC, Ko FW, Ng SS, et al. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010;15:543-50. [Crossref] [PubMed]
- Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol 2021;93:1013-22. [Crossref] [PubMed]
- Mendelson M, Nel J, Blumberg L, et al. Long-COVID: An evolving problem with an extensive impact. S Afr Med J 2020;111:10-2. [Crossref] [PubMed]
- Garg P, Arora U, Kumar A, et al. The "post-COVID" syndrome: How deep is the damage? J Med Virol 2021;93:673-4. [Crossref] [PubMed]
- Stavem K, Ghanima W, Olsen MK, et al. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2021;76:405-7. [Crossref] [PubMed]
- Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun 2020;89:594-600. [Crossref] [PubMed]
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220-32. [Crossref] [PubMed]
- Moreno-Pérez O, Merino E, Leon-Ramirez JM, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J Infect 2021;82:378-83. [Crossref] [PubMed]
- Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int J Environ Res Public Health 2021;18:2621. [Crossref] [PubMed]
- Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: Current State of the Science. Immunity 2020;52:910-41. [Crossref] [PubMed]
- Klok FA, Boon GJAM, Barco S, et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J 2020;56:2001494. [Crossref] [PubMed]
- Hendriks C, Drent M, Elfferich M, et al. The Fatigue Assessment Scale: quality and availability in sarcoidosis and other diseases. Curr Opin Pulm Med 2018;24:495-503. [Crossref] [PubMed]
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. [Crossref] [PubMed]
- Boglione L, Meli G, Poletti F, et al. Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect? QJM 2022;114:865-71. [Crossref] [PubMed]
- Korompoki E, Gavriatopoulou M, Hicklen RS, et al. Epidemiology and organ specific sequelae of post-acute COVID19: A narrative review. J Infect 2021;83:1-16. [Crossref] [PubMed]
- Yang T, Yan MZ, Li X, et al. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: a systematic review and meta-analysis. Infection 2022;50:1067-109. [Crossref] [PubMed]
- Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021;53:737-54. [Crossref] [PubMed]
Cite this article as: Boglione L, Poletti F, Rostagno R, Moglia R, Cantone M, Esposito M, Scianguetta C, Domenicale B, Di Pasquale F, Borrè S. Long-COVID syndrome in hospitalized patients after 2 years of follow-up. J Public Health Emerg 2023;7:4.




