
Modelling forest degradation and risk of disease outbreaks in mainland Equatorial Guinea
Introduction
Emerging infectious diseases (EID) are those that are newly appearing, or which incidence or geographic range are rapidly increasing (1). According to Jones et al. (2008) (2), about 60% of EID are zoonoses, with more than two-thirds of them originating in wildlife [e.g., severe acute respiratory syndrome (SARS) virus, Ebola virus, Nipah virus]. EID may be caused by different types of pathogens including bacteria, protozoans, viruses or fungi and may be transmitted directly or via vectors (3). So-called spillover events can occur where humans, domestic animals and wildlife interact and where pathogens that used to be contained in wild animals infect domestic animals and/or humans. Recent studies on COVID-19 pandemic suggested that its associated coronavirus SARS-CoV-2 has likely originated from bats with subsequent spillovers eventually infecting humans (4). Studies have highlighted a rise in EID and zoonotic spillover events in the tropical areas undergoing ecological and environmental perturbations of their ecosystems (5), with repeatedly identified drivers including deforestation (6), agricultural expansion and other land-use changes (7,8), livestock breeding (9), human settlements in fragmented forest margins leading to increased population densities (10) that facilitated disease transmission (11). Extractive activities such as logging, mining (12), road and dam building (7), irrigation, urbanization (11), as well as bush meat hunting (9,13,14) were also identified as potential drivers. Many of these activities influence the transmission of diseases between wildlife animals and humans but also between wildlife and domestic animals, ultimately affecting human health (15-17).
Several studies have sought to associate different levels of forest degradation to the likelihood of spillover events that could lead to EID. While Faust et al. in 2018 (15) identified areas undergoing intermediate levels of forest degradation as the loci of highest pathogen invasion and probability of individual infection, other scholars linked the emergence of diseases such as Ebola to areas with high levels of dense forest fragmentation (10,18) rather than in deforestation hotspots. Of particular interest are ecotones that are natural or anthropogenic areas of transition between two adjacent ecological systems, for instance between forest and non-forested habitat (19-21). Anthropogenic ecotones, especially, make breeding ground for disease transmission as they concentrate and intensify biophysical factors, biological activity and ecological evolutionary processes (19).
Geospatial analyses have been used to map hotspots of risk of EID emergence at different scales (22,23) or to visualize the distance to these hotspots (18,24). Also, geographical (i.e., physical) accessibility to health care is critical in outbreak control and mitigation efforts, where inadequate access to healthcare services, products or technologies can be responsible for a greater number of cases and deaths as it was for instance the case during Ebola outbreaks (25,26). Thus, a more appropriate approach to appreciate a population’s vulnerability to infectious risks is based on modelling geographical accessibility to health facilities considering realistic travel time of the population, as was undertaken recently on a continental scale by Hulland et al. (27) for areas at-risk of viral hemorrhagic fever.
EID associated to land use change pose a significant threat and public health concern in countries of the tropical area, where deforestation dynamics are currently the greatest (28) and where the presence of dense forest biome and humid climate exacerbate that threat (29). Western Africa and parts of Central Africa have been identified as major hotspots both in terms of EID events, with particular threat from zoonotic pathogens from wildlife and vector borne pathogens (2,22), and forest habitat degradation (30). Central Africa therefore constitutes a relevant case study for the issues related to the links between human health and ecosystem degradation, particularly those triggered by forest habitat loss.
To date, modelling the EID risk through the combination of proximity to hazardous areas (i.e., proximity to deforested areas and ecotones as a proxy for exposure to spillover events) and lack of accessibility to health infrastructures has not been tackled. We aim to fill this gap by proposing a generic approach exemplified with mainland Equatorial Guinea to (I) develop a framework of analyses based on open data to model the EID risk in a context of forest degradation that identifies areas where the population would be most vulnerable to an EID outbreak by incorporating realistic measures of both proximity to potential hazardous areas and accessibility (or lack thereof) to healthcare services, and to (II) apply this workflow to mainland Equatorial Guinea to assess population at risk and provide specific mapping and analysis of areas of interface between forest degradation and anthropic activities, considered potential areas of spillover occurrence. Our goal is to produce a complete reproducible workflow based on high-resolution openly accessible data, and that can be adapted in low- and middle-income countries from the tropical areas to support decision making in land use planning and public health surveillance for risk mitigation. Our proposed methodology is enshrined in the current research momentum that strives to link ecosystem and biodiversity conservation to health (30), with the potential to foster upstream measures decision making and to help target accurate mitigation responses to potential EID risk. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/jphe-20-97).
Methods
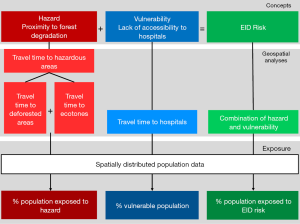
We anchor our methodology on the definition of environmental risk as the “probability and magnitude of consequences after a hazard” (31) and as the intersection of hazard and vulnerability (32). Considering hazards as threats to human communities (33) and vulnerability as the characteristics of a system susceptible to the damaging effect of a hazard (34), our methodology seeks to model spatially-explicit EID risk by combining proximity to disease hazard (hazard hereafter) and vulnerability in terms of accessibility to hospitals. We further model the exposure of the population to levels of hazards, vulnerability and risks by considering the spatially distributed population data. A schematic of the overall methodology is presented in Figure 1. All workflows, as well as information on each data set and detailed data processing are described in the Supplementary file.
Area of study: mainland Equatorial Guinea
Our analyses were applied over the mainland region of Equatorial Guinea where 114.2 kha of tree cover were removed between 2002 and 2019 (35). Deforestation in the country is mainly due to timber exportation towards Asia and Europe and agricultural expansion (32). The country was identified as a potential risk area for yellow fever (36), Ebola and Marburg fever (37,38), and harbors environment suitability for Zika virus (39) although our modelling framework was not designed to identify hotspots of emergence of specific diseases nor to demonstrate the relationship between environmental degradation and disease emergence.
The aforementioned characteristics and the limited surface area of Equatorial Guinea (compared to neighboring countries) made it a relevant case study to achieve our research aims. The area was defined via the borders of Equatorial Guinea from 2018, available on the GADM platform (40).
Statistical analysis
Our analyses consisted in a series of geospatial modelling approaches (detailed in the following sections). First, to obtain exposure to hazards, we obtained areas of deforestation over the period 2010–2014 and areas of anthropic ecotones. We computed the proximity of the population to those hazardous areas, running a travel time accessibility model using a realistic travel scenario. Second, we modelled population vulnerability by running a travel time accessibility model to all hospitals. Third, we modeled the level of EID risk by combining the proximity to hazardous areas with population vulnerability through a normalized index. The population most exposed to EID risk was then obtained through zonal statistics overlaying EID risk with either the spatial distribution of the population or the locations of known settlements. Finally, the interfaces between hazardous areas and several types of anthropic activities were obtained by using zonal statistics tools and raster calculations.
Modelling exposure to hazard through proximity to areas of forest degradation
In order to apprehend the areas of land use change associated with potential spillover risk, we considered two types of hazardous areas associated to forest degradation: deforested areas and ecotones. They were ultimately combined together, and the hazard levels were modelled as the proximity (in terms of travel time) to these hazardous areas. The underlying hypothesis is that that living closer (in terms of travel time) to a hazardous area increases the risk of contracting a zoonotic disease.
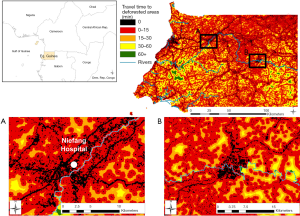
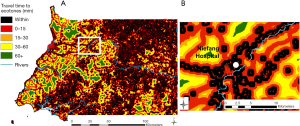
Deforestation was analyzed over periods of five years, as it has been shown that consequent ecological changes can affect epidemiological mechanisms long after deforestation events, depending on the disease [e.g., four (41) or five (42) years after deforestation for malaria, but within the same year for Ebola in (10,18)]. We used the high-resolution Global Forest Watch data sets (28) to get a raster map of forest loss events over the period 2010–2014, at a resolution of 92.52 m. In order to represent areas with frequent interactions and interspecies contact, we modelled anthropic ecotones (19) (ecotones hereafter) considering and combining forest margins and areas of transitional fragmentation, described as “areas of intermediate level of habitat loss” by Faust and colleagues (15). These landscape elements were processed with the Guidos toolbox (43), and result from the combination of a Morphological Segmentation of binary Patterns (MSPA) (44) and a fragmentation analysis (43) using the land cover dataset described here below.
Second, using AccessMod ver. 5 (45), we assembled a 100-m resolution landcover raster from 2015, available on the Copernicus Global Land Service (CGLS) portal (46) to be able to model travel time to hazardous areas. This raster combines landcover information with the road network from 2017 [based on Open Street Map (47) and used in Ouma et al. (48)] and the hydrographic network (rivers and water bodies) from 2018, available on OCHA’s website (49). We constructed a travel scenario targeting the general population moving over the country (see Table S2 in the Supplementary file), assuming motorized transport along the road network, and walking outside of this network. Rivers and water bodies were considered as complete barriers to movement, except in the presence of bridges informed by the road data set. By applying the travel scenario to our combined landcover raster, we obtained a so-called “cost raster”, with each pixel value representing the time to cross that cell depending on the speed of travel, based on a Digital Elevation Model raster at 30-m resolution from 2011, retrieved on the United States Geological Survey (USGS) web platform (50).
Finally, we modelled the levels of hazard across the country by running a travel time analysis using the cost distance function in ArcGIS 10.3 (ESRI, Redlands, USA), considering the time of travelling across the landscape to the edge of the nearest hazardous area. The final step consisted in calculating the percentage of the population within each class of travel time, through Zonal Statistics in ArcGIS, in order to assess the exposure of the population to hazardous areas. For that we used the High Resolution Settlement Layer population dataset from 2018 at 30 m (51).
Modelling population vulnerability through the analysis of accessibility to hospitals
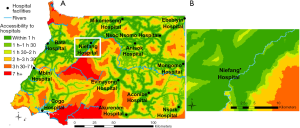
The vulnerability of the population to the infectious risk was translated with an accessibility analysis to the nearest hospital in terms of travel time. We considered the mainland country’s 13 hospitals as of 2017 from Ouma et al. (48), assuming their capacity to manage disease outbreaks, including diagnoses of zoonotic diseases and treatment of affected patients. We used the cost raster obtained in the previous step to model the travel time from any location in the country to the nearest hospital, using AccessMod ver. 5.
The result of this analysis produced a vulnerability map, showing the travel times to the 13 hospitals that was classified into six categories of travel time. We further ran Zonal Statistics analysis in ArcGIS to get the percentage of the population under each travel time category.
Modelling the level of EID risk
The final step of the main workflow consisted in creating an index of the EID risk as the combination of proximity to hazardous areas and the lack of accessibility to hospitals (vulnerability). We first normalized the cost distance raster values and the accessibility raster values, according to the methodology developed by the European Commission for Economic Co-operation and Development (52) which gives:
We then subtracted the normalized raster of accessibility to hospitals to the normalized raster layers of hazard level and represented the results in a heat map classified in five categories of risk, with the highest value expressing the highest EID risk. Risk categories were based on quintiles of the range of risk values based on a geometrical interval classification, which allows one to better visualize the variations of risk throughout the country. This additive approach to model EID risk considers that proximity to hazard and hospital accessibility are likely to compensate each other. However, this approach implies that an area will be considered with a low risk only if the levels of both risk components (hazard and vulnerability) are low.
Finally, in order to quantify the population most exposed to EID risk, we used two complementary approaches: (I) we carried out Zonal Statistics in ArcGIS to get the percentage of the population exposed to EID risk, and (II) we overlaid settlement locations (see Supplementary file: 2. Data sets used in the workflows) on top of the EID risk map, thus producing a map of EID risk exposure of settlements. Note that no population estimates are available for settlements.
The spatial overlap between the EID risk map and population data was carried out considering: (I) both types of hazardous areas (deforested areas and ecotones); (II) hazardous areas linked to deforestation only; (III) hazardous areas linked to ecotones only.
Modelling the interfaces between hazardous areas and anthropic activities
Based on the assumption that forest degradation creates favorable conditions for pathogen spillover between forest matrix and areas of anthropic activities, we aimed to geographically locate potential areas of such event by modelling the interface between hazardous areas and anthropic activities.
Using zonal statistics tools and raster calculations, we modelled the spatial overlap between the hazardous areas and the following anthropic activities over the country (see “3. Detailed methodology” in the Supplementary file for further details): spatial distribution of human population (51), settlements [from 2011, available on the National Geospatial Intelligence Agency website (53)], cropland areas (extracted from the landcover data set), livestock breeding (cattle, goat and sheep densities data combined, using the 1-km resolution raster data sets from the Gridded Livestock of the World (for cattle, goats and sheep) (54-56), and logging concessions [areas of forest logging concessions indexed between 1993 and 2013, available on the Global Forest Watch platform (30)]. These analyses resulted in the proportion of anthropic activities within hazardous areas, highlighting areas that maximize interactions between forest degradation areas and anthropic activities, as well as those areas with conditions likely to trigger pathogen spillover events and epidemic outbreak.
Results
Exposure to hazardous areas
The map of proximity to deforested areas is displayed in Figure 2, and the proximity to ecotones in Figure 3. Results from the zonal statistics indicate that 100% of mainland Equatorial Guinea’s 2018 population was located within less than 15 minutes of travel from a hazardous area. Sixty percent of the population is located within a hazardous area.
Population vulnerability through the analysis of accessibility to hospitals
Figure 4 shows the results of the travel time analysis to the 18 hospitals, highlighting areas where the population would be most vulnerable to an EID hazard due to limited access to the closest hospital. Overlaying the result of this analysis with the distribution of the population in 2018 showed that 92.2% of the population is located within 1 h from the nearest hospital, and that less than 1% (2,717 people) stands very remote (>7 h and up to 13 h 36 min) from the nearest hospital and would be more vulnerable to EID.
EID risk level
Combining travel times to hazardous areas with the vulnerability analysis (travel times to hospitals), we obtained the EID risk level map (Figure 5A). This map allows one to locate “hotspots” of EID risk (in red in Figure 5), which are located in or close to hazardous areas where the accessibility to hospitals is low. EID risks maps are also presented when considering deforested areas only (Figure 5B) and ecotones only (Figure 5C). EID risk hotspots associated to ecotones are a bit more fragmented than the ones using deforested areas only, reflecting the lower number of ecotone items than deforested area.
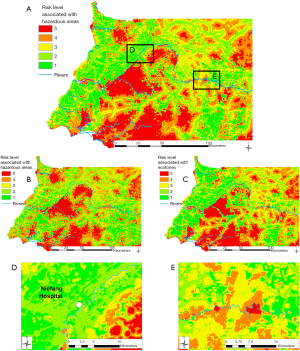
When considering all hazardous areas, we note a low-risk level for the city of Niefang (Figure 5D), which is an example of how the presence of a hospital in Niefang (see Figure 4) contributes to mitigate the high level of hazard associated to the proximity to deforested areas (see Figure 2A) and ecotones (see Figure 3A). On the contrary, the city of Djibloho has no hospital, which translates in a high level of risk as illustrated in Figure 5E.
Results of the Zonal Statistics on all hazardous areas show that only 10.7% of the population of mainland Equatorial Guinea is at medium and high EID risk (levels 3, 4 and 5), while the remaining 89.3% of the population is exposed to low EID risk (levels 1 and 2). When using only hazardous areas linked to deforestation, none of the population is located within the highest risk (level 5), 65.1% is located within areas with a high risk (level 4), 25.0% of the population is located within areas with a medium risk (level 3), and less than 10% of the population is located in low risk (levels 1 and 2) areas. As for the risk associated to the proximity to hazardous areas linked to ecotones only, 4.7% of the population is located within the highest-risk levels 4 and 5, 11.7% within the medium-risk level 3, 71.9% is exposed to risk level 2, and 11.7% within the lowest-risk level 1.
The map of the exposure of settlements to EID risk levels (Figure 6) points to those settlements (53) with the highest risk located close to forest degradation areas and far from a hospital. These are mostly found in the southern and eastern regions of the country, and south to Acoc city, in the middle part of mainland Equatorial Guinea.
Areas of interface between anthropic activities and hazardous areas
Results of the interface analyses are found in Table 1. The high percentage of settlements within hazardous areas (deforested areas and ecotones) highlight human encroachment and dwelling in forest fringes following deforestation events. Cropland and livestock breeding areas within ecotones flag potential areas of increased human and livestock exposure to potential pathogen spillover events.
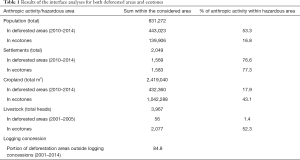
Full table
The interface between logging concessions and deforestation events shows that only a small portion of deforestation can be attributed to logging activities since 84.8% of deforestation between 2001 and 2014 was carried out outside logging concessions.
Discussion
Exposure to hazardous areas and accessibility to hospitals
Comparing the exposure to hazardous areas against the accessibility to hospitals has enabled to point out areas where the population would encounter a greater risk of potential infectious disease emergence and outbreaks, while being far away from the nearest hospital. Complementarily, the overlay of anthropic activities with hazardous areas provides an indication of where spillover events are likely to occur within this area.
Our deforestation mapping pointed out areas of concentrated forest disturbance and loss, and highlights specific, more localized areas likely to create favorable conditions for some pathogen spillover events. Ecotones appear to be more spread out over the territory, with areas of interaction between the remaining fragments or forest core and the anthropic matrix, highlighting areas of potential interactions between humans and wild fauna. Areas with most reduced EID risk combine forest fragments and high accessibility to hospitals and are located mostly on the western and south-eastern parts of the country.
Whereas a classic approach to modelling accessibility would be based on assessing the distance to deforested areas and ecotones, our analyses used more realistic modelling based on travel time combining a motorized and walking transport scenario. Results highlight that for the considered period of time, the major part of the territory was located within 1 h of travel to areas deforested between 2010 and 2014 and to ecotones, which can be explained both by the large area covered by deforestation and ecotones and by the small surface area of the country. Moreover, deforestation areas are often located on or along the main arterial roads resulting in increased exposure to hazardous areas due to greater and easier mobility across the territory.
Accounting for the spatial distribution of the population, our results show that most of the population is located within 1 h of travel from deforested areas or ecotones. At least 60% of the population could be subject to increased monitoring and attention for being located within hazardous areas.
Overlay analyses on the population distribution and the localization of settlements show differing risk exposure levels. We see these two sources of population data as complementary, as the former provides statistical national and subnational population distribution data and its level of exposure, while the latter the spatial localization of populations within mainland Equatorial Guinea accurately pointing out to exposed communities.
Implications for public health, forest management and conservation
We modelled EID risk as the combination of population exposure to hazardous areas and accessibility to hospitals. Our modeling results revealed that most of the population is within 1 h of the nearest hospital, and all of the population within 15 minutes of the nearest hazardous area. The EID risk is thus likely to be mitigated through improvement in forest management, as well as an increased number of infrastructures equipped to monitor, diagnose, and treat the associated infectious diseases.
The EID risk maps enable to localize areas where increased forest degradation in populated areas would result in a significant increase of EID risk due to low accessibility to health infrastructures, thus providing support for measures targeting forest conservation. Our results show that the type of considered hazard (deforested areas/ecotones) will influence the spatial distribution of EID risk and thus the portion of the population exposed to this risk. Moreover, the modelling of interfaces between hazardous areas and anthropic activities can enable to point out the hotspots of increased human or livestock exposure where surveillance measures should be targeted.
Our results highlight that urban expansion and settlements in recently deforested areas should especially be subject to increased monitoring, as a significant part of the population is located within areas which have undergone forest clearance and areas of increased interspecies contact. The mapping of the settlements located in hazardous areas can support decision making for the implementation of monitoring sites.
The EID risk map associated to population distribution and settlements can help localize potential hotspots enabling to identify vulnerable and populated areas where health infrastructures could be implemented or where existing ones could develop proximity outbreak surveillance programs. This is for instance the case in the southern and the north-western parts of the country which lack hospital infrastructures. Anisok and Nsoc Nsomo District hospitals, located in a “high” risk area, could be relevant candidates for surveillance due to their proximity to both types of hazardous areas.
While global hotspots of EIDs have been modelled (2,22,24), there is a need for a more granular approach to EIDs to generate actionable results and support targeted public health interventions by countries. To that aim, our proposed methodology can help prioritize preparedness at a higher spatial resolution. This is in line with the “precision global health” approach (57,58) that seeks, among other goals, to enhance effective resource allocation through the use of high-resolution geospatial data and innovative digital tools.
Limitations and perspectives
Our modelling framework was not designed to identify hotspots of emergence of specific diseases nor to demonstrate the relationship between environmental degradation and disease emergence. Based on the assumption that forest degradation is directly linked to elevated EID risk, our methodology relied on a simple, non-weighted index to describe this risk. Potential future research could test the EID index data generated through this framework against incidence data for different EID within the study area to highlight which diseases are effectively captured by this risk index.
The framework could also be refined by filtering and weighing the hazardous areas according to the factors of emergence for a specific disease (e.g., considering the type of forest cover (18) or the forest patch size (59), or by integrating population movement across the territory (27). Additionally, we recommend that future research look at tailoring this EID risk assessment factor by using socioeconomic, climatic and environmental factors targeted to the subjected disease.
Our geographical accessibility analysis relies on both a road infrastructure data set and a realistic travel scenario. There are however uncertainties on both the exhaustiveness of the road network and the average travel speeds on- and off-roads. This would warrant specific field work in country to lower that uncertainty, which would be particularly important for replication of our methodology in larger countries where this uncertainty can be higher due to heterogeneous mapping throughout the country. Sensitivity analyses on the travel parameters could also be performed to assess how they influence results. Other factors of exacerbation of the infectious disease risk could be included, such as the facilities’ capacity to diagnose and treat specific EID, areas of sustained increase in population densities, or the access to potable water and sanitation (8).
Finally, the temporal disparity between the population data (2018) and the rest of the data sets may have influenced the results of population exposure, considering that deforestation is likely to have increased since 2014.
Conclusions
Our geospatial modelling methodology has combined exposure to hazardous areas with the vulnerability to infectious risks, with the consideration of the known mechanisms of pathogen spillover due to land use change. The translation of anthropic impacts on ecosystems into risk assessment can provide fined-grained territory analysis through realistic and case specific travel scenarios, and we further demonstrated that high-resolution maps to support health and environmental monitoring can be created with open access data, which is replicable in other countries.
Acknowledgments
We would like to thank Paul Ouma for providing formatted hospital and road infrastructure data sets.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/jphe-20-97
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe-20-97). NR serves as an unpaid editorial board member of Journal of Public Health and Emergency from Mar 2020 to Feb 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morse SS. Factors in the Emergence of Infectious Diseases. Emerg Infect Dis 1995;1:7-15. [Crossref] [PubMed]
- Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature 2008;451:990-3. [Crossref] [PubMed]
- World Health Organization. Vector-borne diseases fact sheet [Internet]. 2017 [cited 2020 Apr 20]. Available online: http://www.who.int/mediacentre/factsheets/fs387/en/
- Dhama K, Patel SK, Sharun K, et al. SARS-CoV-2 jumping the species barrier: Zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel Med Infect Dis 2020;37:101830 [Crossref] [PubMed]
- Loh EH, Murray KA, Nava A, et al. Evaluating the Links Between Biodiversity, Land-Use Change, and Infectious Disease Emergence in Tropical Fragmented Landscapes. In: Aguirre AA, Sukumar R. editors. Tropical Conservation: Perspectives on Local and Global Priorities. 1st edition. 2016:79.
- Murray KA, Daszak P. Human ecology in pathogenic landscapes: two hypotheses on how land use change drives viral emergence. Curr Opin Virol 2013;3:79-83. [Crossref] [PubMed]
- Patz JA, Olson SH. Land Use/Land change and Health. In: International Encyclopedia of Public Health [Internet]. Second. Saint Louis: Elsevier Science; 2016. Available online: http://public.eblib.com/choice/publicfullrecord.aspx?p=4718648
- Wilcox BA, Ellis BR. Forests and emerging infectious diseases of humans. Unasylva 2006;57:11-8.
- Pernet O, Schneider BS, Beaty SM, et al. Evidence for henipavirus spillover into human populations in Africa. Nat Commun 2014;5:5342. [Crossref] [PubMed]
- Rulli MC, Santini M, Hayman DT, et al. The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Sci Rep 2017;7:41613. [Crossref] [PubMed]
- Gottdenker NL, Streicker DG, Faust CL, et al. Anthropogenic Land Use Change and Infectious Diseases: A Review of the Evidence. EcoHealth 2014;11:619-32. [Crossref] [PubMed]
- Goldberg TL. Forest Fragmentation as Cause of Bacterial Transmission among Nonhuman Primates, Humans, and Livestock, Uganda. Emerg Infect Dis 2008;14:1375-82. [Crossref] [PubMed]
- Wiethoelter AK, Beltrán-Alcrudo D, Kock R, et al. Global trends in infectious diseases at the wildlife-livestock interface. Proc Natl Acad Sci 2015;112:9662-7. [Crossref] [PubMed]
- Wolfe ND, Eitel MN, Gockowski J, et al. Deforestation, hunting and the ecology of microbial emergence. Glob Change Hum Health 2000;1:10. [Crossref]
- Faust CL, McCallum HI, Bloomfield LSP, et al. Pathogen spillover during land conversion. Ecol Lett 2018;21:471-83. [Crossref] [PubMed]
- Bloomfield LSP, McIntosh TL, Lambin EF. Habitat fragmentation, livelihood behaviors, and contact between people and nonhuman primates in Africa. Landsc Ecol 2020;35:985-1000. [Crossref]
- Gibb R, Redding DW, Chin KQ, et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature 2020;584:398-402. [Crossref] [PubMed]
- Olivero J, Fa JE, Real R, et al. Recent loss of closed forests is associated with Ebola virus disease outbreaks. Sci Rep 2017;7:14291. [Crossref] [PubMed]
- Despommier D, Ellis BR, Wilcox BA. The Role of Ecotones in Emerging Infectious Diseases. EcoHealth 2007;3:281-9. [Crossref]
- Cain ML, Bowman WD, Hacker SD. Ecology. 3rd edition. Sunderland, Massachusetts, USA: Sinauer Associates, Inc., 2014.
- Fonseca MS. Edge Effects. In: Jørgensen SE, Fath BD. editors. Encyclopedia of ecology. 1st edition. Amsterdam; Boston: Elsevier, 2008.
- Allen T, Murray KA, Zambrana-Torrelio C, et al. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun 2017;8:1124. [Crossref] [PubMed]
- Valle D, Tucker Lima JM. Large-scale drivers of malaria and priority areas for prevention and control in the Brazilian Amazon region using a novel multi-pathogen geospatial model. Malar J 2014;13:443. [Crossref] [PubMed]
- Morris AL, Guégan JF, Andreou D, et al. Deforestation-driven food-web collapse linked to emerging tropical infectious disease, Mycobacterium ulcerans. Sci Adv 2016;2:e1600387 [Crossref] [PubMed]
- Heymann DL, Chen L, Takemi K, et al. Global health security: the wider lessons from the west African Ebola virus disease epidemic. Lancet 2015;385:1884-901. [Crossref] [PubMed]
- Gostin LO, Friedman EA. A retrospective and prospective analysis of the west African Ebola virus disease epidemic: robust national health systems at the foundation and an empowered WHO at the apex. Lancet 2015;385:1902-9. [Crossref] [PubMed]
- Hulland EN, Wiens KE, Shirude S, et al. Travel time to health facilities in areas of outbreak potential: maps for guiding local preparedness and response. BMC Med 2019;17:232. [Crossref] [PubMed]
- Hansen MC, Potapov PV, Moore R, et al. High-resolution global maps of 21st-century forest cover change. Science 2013;342:850-3. [Crossref] [PubMed]
- Colfer CJP, Sheil D, Kaimowitz D, et al. Forest and human health in the tropicals: some important connections. Unasylva 2006;57:3-10.
- Global Forest Watch. Interactive Map [Internet]. 2018 [cited 2020 Apr 20]. Available online: https://www.globalforestwatch.org/map/4/27.92/57.04/ALL/grayscale/none?tab=analysis-tab&dont_analyze=true
- Turner BL, Kasperson RE, Matson PA, et al. A framework for vulnerability analysis in sustainability science. Proc Natl Acad Sci 2003;100:8074-9. [Crossref] [PubMed]
- Escobar-Wolf R, Bouali EH, Oommen T. Risk Assessment. In: Bobrowsky PT, Marker B. editors. Encyclopedia of Engineering Geology. Cham: Springer International Publishing, 2018:758-61.
- UN ISDR. Environment and Vulnerability. Emerging Perspectives [Internet]. Geneva, Switzerland: UNEP, 2011 [cited 2020 Jul 20]. Available online: https://postconflict.unep.ch/download/26_april/UNEP_ISDR_Environment_and_Vulnerability.pdf
- UN ISDR. Terminology on Disaster Risk reduction. [Internet]. Geneva, Switzerland: United Nations, 2009 [cited 2020 Jul 24]. Available online: https://www.preventionweb.net/files/7817_UNISDRTerminologyEnglish.pdf
- Central African Forest Initiative. Equatorial Guinea [Internet]. [cited 2018 Apr 20]. Available online: http://www.cafi.org/content/cafi/en/home/partner-countries/equatorial-guinea.html
- Shearer FM, Longbottom J, Browne AJ, et al. Existing and potential infection risk zones of yellow fever worldwide: a modelling analysis. Lancet Glob Health 2018;6:e270-8. [Crossref] [PubMed]
- Pigott DM, Golding N, Mylne A, et al. Mapping the zoonotic niche of Ebola virus disease in Africa. Elife 2014;3:e04395 [Crossref] [PubMed]
- Pigott DM, Deshpande A, Letourneau I, et al. Local, national, and regional viral haemorrhagic fever pandemic potential in Africa: a multistage analysis. Lancet 2017;390:2662-72. [Crossref] [PubMed]
- Messina JP, Kraemer MU, Brady OJ, et al. Mapping global environmental suitability for Zika virus. Elife 2016;5:e15272 [Crossref] [PubMed]
- GADM. Download GADM Data. [Internet]. GADM, 2018. Available online: https://gadm.org/download_country_v3.html
- Wayant NM, Maldonado D, Rojas de Arias A, et al. Correlation between normalized difference vegetation index and malaria in a subtropical rain forest undergoing rapid anthropogenic alteration. Geospat Health 2010;4:179-90. [Crossref] [PubMed]
- Fornace KM, Abidin TR, Alexander N, et al. Association between Landscape Factors and Spatial Patterns of Plasmodium knowlesi Infections in Sabah, Malaysia. Emerg Infect Dis 2016;22:201-8. [Crossref] [PubMed]
- Vogt P. Measuring Forest Spatial Pattern with mathematical morphology. (Available in the free JRC software GuidosToolbox). [Internet]. 2018 [cited 2020 Apr 20]. Available online: http://ies-ows.jrc.ec.europa.eu/gtb/GTB/psheets/GTB-Pattern-Morphology.pdf
- Soille P, Vogt P. Morphological segmentation of binary patterns. Pattern Recognit Lett 2009;30:456-9. [Crossref]
- Ray N, Ebener S. AccessMod 3.0: computing geographic coverage and accessibility to health care services using anisotropic movement of patients. Int J Health Geogr 2008;7:63. [Crossref] [PubMed]
- Jacobs T, Smets B. Moderate Dynamic Land Cover, Vegetation and Energy. [Internet]. CGLOPS Copernicus Global Land Operations, 2017 [cited 2018 Apr 24]. Available online: http://copernicus.eu
- Open Street Map. Equatorial Guinea [Internet]. 2018. Available online: http://download.geofabrik.de/africa/equatorial-guinea-latest-free.shp.zip
- Ouma PO, Maina J, Thuranira PN, et al. Access to emergency hospital care provided by the public sector in sub-Saharan Africa in 2015: a geocoded inventory and spatial analysis. Lancet Glob Health 2018;6:e342-50. [Crossref] [PubMed]
- OCHA Services. Equatorial Guinea [Internet]. HDX. 2018 [cited 2018 May 1]. Available online: https://data.humdata.org/group/gnq?
- US Department of the Interior. Earth Explorer [Internet]. USGS. 2011 [cited 2018 Apr 15]. Available online: https://earthexplorer.usgs.gov/
- Facebook Connectivity Lab, Center for International Earth Science Information Network - CIESIN. High Resolution Settlement Layer (HRSL). Source imagery for HRSL ©. Columbia University. Digital Globe, 2016.
- Joint Research Centre-European Commission. Handbook on constructing composite indicators: methodology and user guide. Paris: OECD, 2008.
- National Geospatial Intelligence Agency. Equatorial Guinea Settlements [Internet]. Humanitarian Data Exchange, 2011. Available online: https://data.humdata.org/dataset/equatorial-guinea-settlements
- Robinson T, Conchedda G. GLW 2 - Gridded Livestock of the World 2 - Global Distributions of Cattle (Cattle Global) [Internet]. geo-wiki; 2014. Available online: https://livestock.geo-wiki.org/download
- Robinson T, Conchedda G. GLW2 - Gridded Livestock of the World 2 - Global Distribution of Goats (Goats Global). [Internet]. geo-wiki; 2014. Available online: https://livestock.geo-wiki.org/download/
- Robinson T, Conchedda G. GLW2 - Gridded Livestock of the World 2 - Global Distribution of Sheep (Sheep Global) [Internet]. geo-wiki; 2014. Available online: https://livestock.geo-wiki.org/download/
- Sheath DJ, Ruiz de Castañeda R, Bempong NE, et al. Precision global health: a roadmap for augmented action. J Public Health Emerg 2020;4:5. [Crossref]
- Flahault A, Utzinger J, Eckerle I, et al. Precision global health for real-time action. Lancet Digit Health 2020;2:e58-9. [Crossref] [PubMed]
- Chaves LSM, Conn JE, López RVM, et al. Abundance of impacted forest patches less than 5 km2 is a key driver of the incidence of malaria in Amazonian Brazil. Sci Rep 2018;8:7077. [Crossref] [PubMed]
Cite this article as: Poirier O, Ruiz de Castañeda R, Bolon I, Ray N. Modelling forest degradation and risk of disease outbreaks in mainland Equatorial Guinea. J Public Health Emerg 2021;5:15.



