
Analysis of laboratory findings in recovered and non-recovered patients with COVID-19: a longitudinal observational study in Jilin Province of China
Introduction
Coronavirus disease 2019 (COVID-19) has caused an epidemic throughout the world since December, 2019. Although some researchers have established the clinical and/or epidemiologic features of the patients with COVID-19 (1,2), and a few researchers focus on the longitudinal observational study in T cell counts, cytokine levels and IgM, IgA and IgG SARS-CoV-2 antibodies in the patients with COVID-19 (3,4). The insightful clues have been confirmed to be revealed by longitudinal change patterns of laboratory tests on disease progression and recovery progression (5). Therefore, it is important to analyze and summarize longitudinal laboratory characteristics for monitoring and evaluating patients with COVID-19.
This research aimed to investigate epidemiological, the laboratory characteristics and outcomes of patients with COVID-19, including differences of laboratory data between recovered and non-recovered patients. We hope our study will provide useful prognostic factor from laboratory findings for accurate individualized assessment of COVID-19. We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/aoe-20-72).
Methods
Patient selection and data sources
This study was a multi-center study focusing on the patients with a laboratory confirmed COVID-19, which included 68 patients in the First Hospital of Jilin University (n=4), Siping Infectious Disease Hospital (n=22) or Changchun Infectious Disease Hospital (n=42) in Jilin Province, China, from January 21, 2020 to February21, 2020. Epidemiological, demographic, date of illness onset, hospital admission date, laboratory findings, outcomes data, and severity of COVID-19 with data collection forms, were collected from electronic medical records system. Two researchers separately evaluated the record collection forms to ensure the accuracy of the record. The onset day was described as the date when any symptoms were observed by the patients. Severity of COVID-19 was described according to the diagnostic and treatment guideline for SARS-CoV-2 issued by Chinese National Health Committee (Version 3-5). Severe COVID-19 was confirmed if the patients had one of the following criteria: (I) respiratory distress with respiratory frequency ≥30/min; (II) pulse oximeter oxygen saturation ≤93% at rest; (III) oxygenation index (artery partial pressure of oxygen (PaO2)/inspired oxygen fraction (FiO2) ≤300 mmHg. The clinical outcomes were continuously tracked until February 21, 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of the First Hospital of Jilin University (No. 2020-313), the Ethics Committee of Changchun Infectious Disease Hospital (No. 2020-001), and the Ethics Committee of Siping Infectious Disease Hospital (No. 2020-001). Informed consent of all subjects was waived.
Laboratory tests
All the suspected patients with COVID-19 were taken sputum and throat swab specimens at admission and stored in virus transport medium, which were transported to local Center for Disease Control and Prevention (Changchun or Siping). Doubtful positive specimens were taken to determine again by Jilin Provincial Center for Disease Control and Prevention. Specimens were determined by real time reverse-transcription-polymerase-chain-reaction (RT-PCR) for SARS-Cov-2 RNA within three hours. Virus examination was repeated twice every 24 hours. The assay was carried out by SARS-CoV-2 nucleic acid detection kit based on the manufacturer’s protocol (Shanghai bio-germ Medical Technology Co. Ltd, and Shanghai GeneoDx Biotech Co. Ltd). Patients with COVID-19 were rehabilitateed from hospital once the results of two RT-PCR tests at 24-hour intervals were negative for SARS-Cov-2. Laboratory tests, including haematological, biochemical, and immunological tests, were carried out at admission. All renewed results for laboratory data during hospitalization were collected as disease progressed. The laboratory findings for certain patients were missing because the absence of kinds of tests or delayed results. All reagents related to test were original from same manufacturer, which means that the reagent and analyzer are matched.
All clinicians, technicians, and nurses participating in the epidemic had been appropriately trained. In 2012, reference interval (RI) standards for common biochemical analyte and blood cell analysis in Chinese adults were published by the Ministry of Health of the People’s Republic of China (6,7). The RIs of tests were all performed by these Standards in the three designated hospitals. The RI of troponin I was below the 99th percentile of healthy people.
Statistical analysis
The clinical characteristics such as age, recovery time, sex and the laboratory tests at the baseline line stratified by the recovery status are presented as median (IQR) for continuous variables and as n (%) for categorical variables.
Here, the hospital day is divided into four periods: the first period on the day of admission, the second period ranges from the second day to the fifth day, the third period from the sixth day to the tenth day, and the fourth period after the tenth day. For the longitudinal laboratory measurement profiles, the Generalized Estimating Equation (GEE) models (8) were fit separately for each parameter to examine if there were different change patterns between the recovered group and the non-recovered group, with the hospital day indicators, the recovery status and their interaction terms as covariates and the correlation structure matrix as unstructured type. Upon those tests with either the absolute robust Z score of the recovery status indicator (meaning there is a difference between the two groups at the baseline) or those of at least one interaction term (meaning there might be a difference between the two groups during the hospitalized days) is larger than 1.96, and 1,000 bootstrapped replicates were made to construct the 95% confidence intervals (CIs) of estimated values for individual period and recovery strata.
Lastly, a multiple logistic regression model with the recovery status as the response variable and patients’ age, sex, severity type and laboratory tests that differed significantly at the baseline as covariates was fit, and a nomogram was diagramed. All analyses were performed by the R language, version 3.6.1 (www.r-project.org).
Results
Demographics and exposures
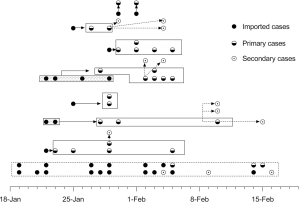
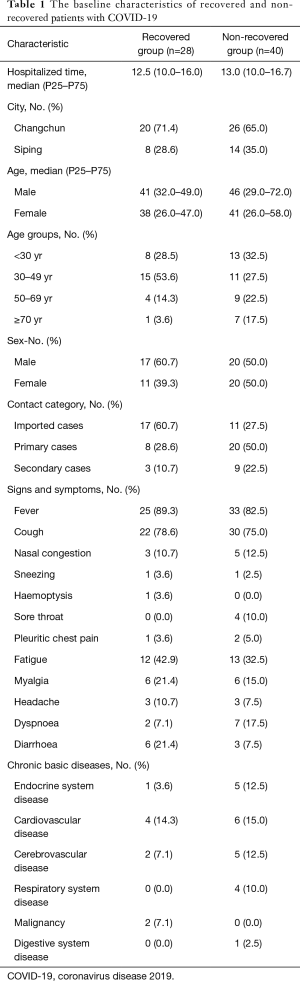
Of the 68 patients with COVID-19 in this study, 63 were diagnosed as mild and 5 as moderate or severe on admission. The median age of the patients was 41 years (range, 8–87 years), and 30.9% of them were more than 50 years old. Thirty-one (54.4%) of patients were male. The median intervals from the onset of symptoms to hospital admission for all patients were 4 days (IQR, 1–7 days). As of the termination day of this study, 28 subjects were rehabilitated (recovered group) and 40 patients were admitted still (non-recovered group). The median hospitalized time were 12.5 days (IQR, 10.0–16.0 days) for recovered patients and 13.0 days (IQR, 10.0–16.7 days) for non-recovered subjects. The median age of the two groups was not statistically different, and the recovered group and the non-recovered group were 41 years old and 42.5 years old, respectively. None of the 68 patients had a history of Huanan seafood market exposure in Wuhan, 28 of them (41.2%) were imported cases. We define imported cases as patient who had been diagnosed as COVID-19 and had travel history in Hubei Province or contact history with COVID-19 patients outside Jilin Province within 14 days before the onset of symptoms. Here, 28 of them (41.2%) were primary cases, who stayed in Jilin Province without leaving but had a close exposure history with imported patients, and the other 12 (17.6%) patients were secondary cases, who had a close exposure history with primary contacts. The transmission route for COVID-19 patient is summarized in Figure 1. The baseline characteristics of recovered and non-recovered patients with COVID-19 is presented in Table 1.
Full table
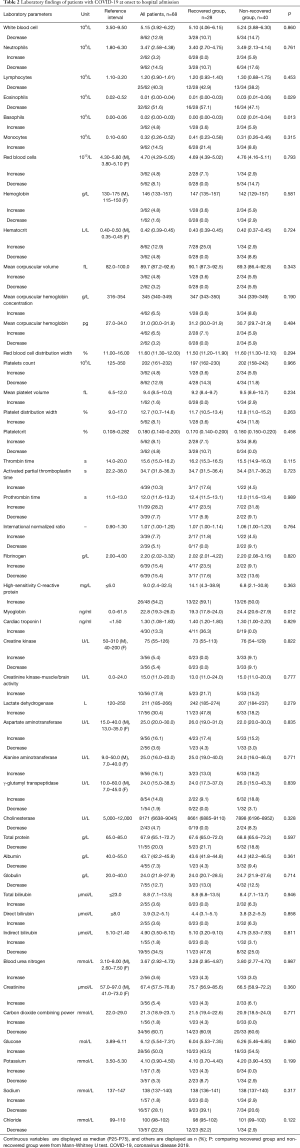
For leukocyte parameters, white blood cell, neutrophils (NE), lymphocytes (LY), and eosinophils (EO) were below the lower reference limit (LRL) in 12.9%, 14.5%, 40.3%, and 51.6% patients, while basophils (BA) and monocytes were higher than upper reference limit (URL) in 4.8% and 14.5% patients at onset to hospital admission, respectively. The values of EO and BA in recovered patients were lower than non-recovered patients, respectively (P<0.05). For erythrocyte parameters, red blood cells (RBC), hemoglobin (HGB), and hematocrit (HCT) were below the LRL in 8.1%, 1.6%, and 4.8% patients, respectively. For Platelet parameters, decreased platelets count (PLT) was found in 12.9% patients. For coagulation parameters, activated partial thromboplastin time (APTT), prothrombin time (PT), and international Normalized Ratio were increased in 10.3%, 28.2% and 7.7% patients, respectively, while fibrinogen were decreased in 15.4% patients. For serum cardiac markers, aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine kinase (CK), creatinine kinase-muscle/brain activity (CK-MB), and cardiac troponin I (cTnI), are higher than the URL in 16.1%, 30.4%, 5.4%, 17.9%, and 13.3% patients, respectively. For liver function tests, increased alanine aminotransferase (ALT), γ-glutamyl transpeptidase, total bilirubin, direct bilirubin, and indirect bilirubin were found in 16.1%, 14.8%, 3.6%, 3.6% and 1.8% patients, while cholinesterase (CHE), total protein, albumin and globulin (GLB) were decreased in 4.7%, 20.0%, 7.3%, and 12.7% patients, respectively. For kidney function tests, blood urea nitrogen and creatinine were increased in 3.6%, and 5.4% patients, respectively. For electrolyte tests, potassium, sodium and chloride were decreased in 5.3%, 28.1% and 22.8% patients, respectively. Laboratory data of patients with COVID-19 at onset to hospital admission are shown in Table 2.
Full table
Temporal trajectories of laboratory tests
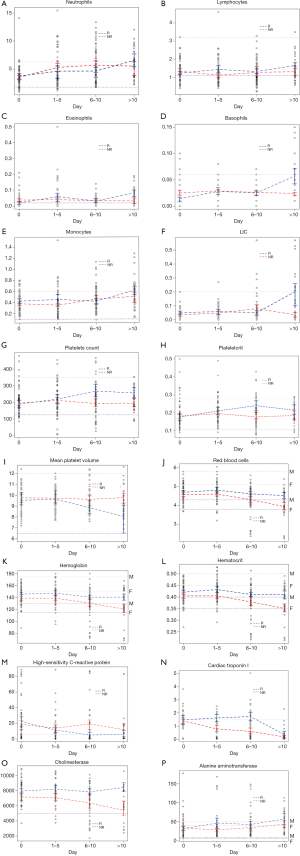
LIC, an immature leukocyte subpopulation, was significantly increased after day 10 in recovered patients as well as increased trends in NE, LY, EO, BA and MO. Platelet parameters—PLT, plateletcrit (PCT) and mean platelet volume (MPV), indicators of platelet function in vivo, were in the range of RI in most cases and no difference before day 5 in recovered and non-recovered patients. Interestingly, PLT and PCT were significantly increased after day 6 in recovered patients, while MPV was reduced markedly from day 6 in recovered patients, even close to the LRL. For erythrocyte parameters—RBC, HGB, and HCT, no statistical differences before day 10 were found between recovered and non-recovered patients. All these three parameters were non-marginally higher after day 10 in recovered patients, while the parameters showed gradual decline, especially from day 6 below the LRL in non-recovered patients.
CRP, a marker of inflammation, was elevated at onset in both groups, and it declined to the URL at day 6 and remained constant afterwards in recovered patients, compared with always higher levels than the URL in non-recovered patients. Serum cardiac markers—AST, LDH, CK, CK-MB, and cTnI, are higher than the URL in 16.1%, 30.4%, 5.4%, 17.9%, and 13.3% patients at onset to hospital admission, respectively. Especially, cTnI that is a marker of myocardial injury was always higher from onset to recovery in recovered patients, yet it was sharply declined below the URL after day 10. CHE, an enzyme that reflects hepatic synthetic function, was always higher during the recovery process in recovered patients than in non-recovered patients. However, ALT, another enzyme in liver cells, was always slightly elevated over the URL in recovered patients than in non-recovered patients, indicating gently liver injury even disease recovery. Compared with non-recovered patients, GLB was lower at all time points, suggesting no strong immune response in these patients. Comparison of laboratory findings on the day of admission in recovered and non-recovered patients is shown in Table 2 and their change trajectories over time are presented in Figure 2.
Predictors of recovery
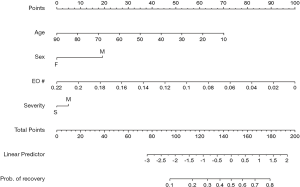
According to the GEE analysis and the baseline comparison (as shown in Figure 2), both EO and BA differed at the first measurement on the day of admission in recovered and non-recovered patients, and they had similar change trajectories over time. Since EO and BA are highly positively correlated at the baseline, BA was discarded from the downstream analysis to avoid the co-linearity issue, and a multiple logistic regression model was fit with EO level at the baseline, age, severity level and gender as predictors to estimate the possibility of recovery for the patients with COVID-19. Both age (P=0.041) and EO (P=0.025) were identified as independent predictors for recovery. The corresponding nomogram was made to elucidate graphically the association of these predictors with recovery (Figure 3). As shown in Figure 3, a young patient had a better chance to recover from the disease than an elderly patient. If a patient had a smaller EO value at the baseline, the probability of recovery was higher.
Discussion
Most patients with COVID-19 were middle and elderly aged. SARS-CoV-2 infection was proved by RT-PCR in all patients. There are 28 patients in recovered group and 40 patients in non-recovered group in the current study, respectively. The median age of the two groups was not statistically different. Of the 68 patients, 41.2% were imported cases-patients, 41.2% were primary cases, and 17.6% were secondary cases. In this study, severe ill ones are all over 45 years old. We proposed that EO and age may be a potential predictor.
Haematological tests
In this study, eosinopenia was found in 51.6% patients at onset to hospital admission, consistent with other recent report (9). The recovered patients had significantly lower EO levels than the non-recovered patients at onset. However, EO level was significantly increased after day 10 in recovered patients as well as BA. Therefore, the trend of EO may be used as a recovery indicator for COVID-19. Eosinopenia may be a crucial diagnostic clue in the patients with typical symptoms and radiological changes with and without lymphopenia. In those confirmed patients with COVID-19, continuously increased EO and BA level after treatment may be a sign for beginning recovery. The pathophysiology for eosinopenia in COVID-19 remains uncertain but is likely associated with multiple factors, involving inhibition of EO egress from the bone marrow, blockade of eosinophilopoiesis, decreased expression of chemokine receptors/adhesion factors and/or direct EO cell death induced by type 1 interferons released during the acute infection (10).
The absolute value of LY in 40.3% patients was decreased at onset to hospital admission in this study, between 35% and 75.4% in previous studies (9,11). Lymphopenia indicates that SARS-CoV-2 might primarily work on LY, as does SARS-CoV. LY, including immature leukocyte subpopulation, were significantly increased after day 10 in recovered patients, predicting increased leukocyte release and improved immune response after effective treatment in recovered patients.
PLT were below the LRL in 12.9% patients at onset to hospital admission, consistent with the report of 12% patients (n=99) in certain hospital (11), and lower than the study of 36.2% patients (n=1,099) in 552 hospitals, China (12). The abnormalities of these laboratory indexes are more obvious in critically ill cases. In our previous research (13), the possible mechanisms of thrombocytopenia in the patients with COVID-19 are as following: (I) The virus directly infects bone marrow stromal cells, inducing cell apoptosis and growth inhibition. (II) Platelets are damaged by the immune system. (III) Platelets aggregates and forms microthrombus, resulting in increased consumption in lung. In this study, PLT and PCT are significantly increased and MPV is decreased sharply in most cases within the range of RI after day 6 in recovered patients. The changes of these parameters suggested platelet parameters are probably indicative indexes for recovered patients.
RBC, HGB, and HCT were mostly in the range of RI in the whole course of disease and were non-marginally higher after day 10 in recovered patients. However, the three parameters showed gradual decline, especially from day 6 below the LRL in non-recovered patients. A recent study showed the ORF8 and surface glycoprotein may respectively bind to the porphyrin, while orf1ab, ORF10 and ORF3a proteins could coordinately attack heme to dissociate the iron to form the porphyrin (14). The mechanism heavily disturbed with the normal heme anabolic pathway of the human body. Moreover, chloroquine could prevent orf1ab, ORF3a and ORF10 to attack the heme to form the porphyrin, and inhibit the binding of ORF8 and surface glycoproteins to porphyrins to some extent. In the present study, the changes of these parameters indicate that enough RBC and HGB can improve the recovery of recovered patients.
Biochemical and immunological tests
A study reported COVID-19 patients had a great deal of proinflammatory cytokines including IL1B, IFNγ, IP10, and MCP1, probably leading to activated T-helper-1 (Th1) cell responses (15). In this study, marker of inflammation-CRP was elevated at onset to hospital admission in both groups as well as serum ferritin (4 cases in the First Hospital of Jilin University, data not show), serum procalcitonin (1 case in Siping Infectious Disease Hospital, data not show), and erythrocyte sedimentation rate (8 cases in Siping Infectious Disease Hospital, data not show). Moreover, CRP continued to decline to the LRL at day 7 in recovered patients, compared with always higher levels than the URL in non-recovered patients. This study suggested that the cytokine storm was related to recovery process.
A study confirmed that about 12% patients with COVID-19 subjected acute cardiac injury (15). Subjects with cardiac disease had a higher fatality rate. In this study, serum cardiac markers—AST, LDH, CK, CK-MB, and cTnI, were higher than the URL in 5.4% to 30.4% patients at onset to hospital admission, respectively. Moreover, cTnI is always higher from onset to recovery in recovered patients, yet it is sharply declined below the URL after day 10. Therefore, the serum cardiac markers of in patients with COVID-19 are important to evaluate heart function.
Hepatic injury has been observed patients among COVID-19 (16). In this study, CHE is always higher during the process in recovered patients than in non-recovered patients. However, ALT is always slightly elevated over the URL in recovered patients than in non-recovered patients, indicating gently liver injury in the process of disease recovery. In contrast with non-recovered patients, GLB is lower at all time points, suggesting no strong immune response in recovered patients.
Limitations of this study
There were some limitations in the study. First, 68 patients were enrolled in this study and the number of samples was limited by region. Second, for the main objective in the present study was to discuss the temporal trajectories of laboratory results, the data of clinical manifestation of patients with COVID-19 was absent.
Conclusions
By evaluating the temporal trajectories of laboratory markers, we found that many had different change patterns between the recovered and non-recovered patients. Especially, EO may serve as an independent predictor for recovery in addition to age. The monitoring of the dynamic level of markers can give more effective clues for the judgment of the progress in COVID-19 patients. Since COVID-19 is a new type of infectious disease, people know little about it so far. This study can help decipher the disease and pinpoint effective treatment regimen from the view of laboratory tests which are more inexpensive and routine compared to nucleic acid testing and CT. It will be necessary to confirm this data in larger samples, without excluding the need for RT-PCR.
Acknowledgments
Funding: This work was supported by grants from Jilin Science and Technology Development Program (No. 20170623092TC-09; No. 20190304110YY), The First Hospital Translational Funding for Scientific &Technological Achievements (No. JDYYZH-1902002).
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/jphe-20-82
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jphe-20-82
Peer Review File: Available at http://dx.doi.org/10.21037/jphe-20-82
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe-20-82). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Hospital of Jilin University (No.: 2020-313), the Ethics Committee of Changchun Infectious Disease Hospital (No.: 2020-001), and the Ethics Committee of Siping Infectious Disease Hospital (No.: 2020-001) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020.102433. [Crossref] [PubMed]
- Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging 2020;47:1275-80. [Crossref] [PubMed]
- Padoan A, Sciacovelli L, Basso D, et al. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin Chim Acta 2020;507:164-6. [Crossref] [PubMed]
- Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020;55:102763. [Crossref] [PubMed]
- Tian SY, Zhu XT, Sun XJ, et al. A Prognostic Model to Predict Recovery of COVID-19 Patients Based on Longitudinal Laboratory Findings. Virol Sin 2020.811-9. [PubMed]
- Chinese National Health Committee. Reference intervals for blood cell analysis. WS/T 405-2012. National Health Committee of the People's Republic of China. Available online: http://www.nhc.gov.cn/wjw/s9492/wsbz_3.shtml. Accessed March 2 2020.
- Chinese National Health Committee. Reference intervals for common clinical biochemistry tests. WS/T 404-2012. National Health Committee of the People's Republic of China. Available online: http://www.nhc.gov.cn/wjw/s9492/wsbz_4.shtml. Accessed March 2 2020.
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13-22. [Crossref]
- Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730-41. [Crossref] [PubMed]
- Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil Responses During COVID-19 Infections and Coronavirus Vaccination. J Allergy Clin Immunol 2020;146:1-7. [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 30;382:1708-20.
- Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol 2020;99:1205-8. [Crossref] [PubMed]
- Wenzhong L, Hualan L. COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism. ChemRxiv. Preprint 2020. Available online: https://doi.org/ [Crossref]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
Cite this article as: Tian S, Sun X, Wang J, Zhu X, Liu K, Yan F, Wu M, Zhang X, Wang Q, Xu J. Analysis of laboratory findings in recovered and non-recovered patients with COVID-19: a longitudinal observational study in Jilin Province of China. J Public Health Emerg 2021;5:4.


