
Small ubiquitin-like modifier 1 gene associated with noise-induced hearing loss in Chinese workers
Introduction
NIHL is an important category of hearing loss, which is primarily caused by continuous or intermittent noise expose for a long time (1), and usually develops slowly within a few years (2). In the world, the hearing loss of adults caused by occupational noise accounts for 16% (3). At present, more than 10 million employees in China work in environments with excessive noise levels, and millions of them suffer from hearing impairment to varying degrees (4). Noise is not the only cause of NIHL. Environmental factors such as smoking, drinking, exposure to organic solvents are also risk factors for NIHL (5,6). Genetic are also factors of consequence in the occurrence and development of NIHL. Single nucleotide polymorphisms (SNPs), as one of the most common human heritable variants, refers to the polymorphism of DNA sequence caused by single nucleotide variation at the genomic level. The studies of Ding and Xu found that the SNPs of OGG1, APEX1, XRCC1 and PCDH15 genes is related to human NIHL susceptibility (7,8). To comprehend the pathogenesis of NIHL, more genetic epidemiology studies still need to explore the genes related to NIHL susceptibility.
SUMO (small ubiquitin-related modifier) can enhance the stability of proteins or regulate the location and distribution of proteins in cells, as well as affect the transcriptional activity of proteins. The small ubiquitin-like modifier 1 (SUMO-1) is reported to be a critical member of the ubiquitin-related protein family. Through binding to intracellular target proteins, SUMO-1 is an essential component of the post-translational modification system, which participates in numerous of cellular biological processes, covering nuclear transport, transcriptional regulation, cell apoptosis, and protein stability. Many studies have found that the imbalance of SUMOylation or deSUMOylation processes of protein is related to the occurrence of many diseases and even cancers (9-11). For example, SUMO-1 siRNA down-regulated H4 SUMOylation, Keep the endometrial cancer cells from proliferation and induces apoptosis (12). Prostaglandin E2 (PGE2) enhances the proliferation and invasion of endometrial cancer cells by increasing SUMO-1, through EP4 receptors (13). Ginkgolic Acid is a SUMO-1 inhibitor, which can inhibit the progression of oral squamous cell carcinoma by reducing the SUMOylation of SMAD4 (14). And silencing SUMO-1 gene can reduce the proliferation of gastric cancer cells (15). However, there are none report about the relationship between SUMO-1 gene polymorphism and NIHL susceptibility. Therefore, we propose the hypothesis that the polymorphism of SUMO-1 gene may be related to the pathogenesis and susceptibility to the NIHL. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jphe-20-114).
Methods
Patient selection
A retrospective case/control study was conducted to find the relationship between SUMO-1 SNP and NIHL susceptibility. In this current research, 2,689 workers who were exposed to occupational mechanical noise in one plant in Jiangsu province, east China were gathered as objects of study in 2015. Chinese occupational NIHL diagnostic criteria (GBZ49-2014) were then used to identify subjects with hearing loss or not. Occupational noise exposure in this research is defined as exceeding 85 dB (A) within 8 hours of a working day. Case definition and control definition were assigned as follows, case group: average binaural high-frequencies in 3,000, 4,000, and 6,000 Hz hearing thresholds were >25 dB (25 dB not included), or they were ≤25 dB but with the worse ear’s speech frequencies in 500, 1,000, and 2,000 Hz hearing threshold were >25 dB (25 dB not included). In the control group, the average binaural high-frequencies hearing threshold were ≤25 dB with the worse ear’s speech frequencies hearing thresholds were ≤25 dB. According to the exclusion criteria (a. subjects with missing data, b. subjects without blood collection, c. subjects with ototoxic drug administration were excluded). Finally, 586 cases and 639 controls were included in this study, matching sex, age, smoking, drinking, noise working time and noise exposure intensity. Study size: all subjects accord with screening criteria were included in this study. All data (questionnaire, pure-tone audiometry, measurement of ambient noise, temperature) in current research were collected by trained investigators following relevant pipelines and regulations. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study obtained the informed consent of all participants and was approved by the Ethics Committee of Jiangsu Center for Disease Control and Prevention [2014029].
Questionnaire investigation
The questionnaire content mainly includes: the general demographic characteristics (such as gender, age and education level), the histories of diseases affecting hearing (head trauma, history of use of ototoxic drugs, etc.), living habits (history of smoking and drinking). Smoking status in this study was split into three groups: smoking group, ever smoking group and never smoking group. Drinking status was also split into three groups: drinking group, ever drinking group and never drinking group. If a person smokes a cigarette or more or drinks fifty grams of alcohol or more (spirits and beer) in a day for more than a year, he or she was assigned into a smoking or drinking group. If a participant has quit smoking or drinking for more than a year, he or she was assigned to ever smoking group or ever drinking group.
Pure-tone audiometry
Subjects should avoid exposure to noise (>85 dB) for at least twelve hours before accepting pure tone audiometry. Then 500, 1,000, 2,000, 3,000, 4,000, and 6,000 Hz pure tone air threshold tests were performed by otorhinolaryngologist in the sound attenuation room. In accordance with the Chinese Diagnostic criteria of Occupational noise-induced hearing loss (NIHL), the binaural hearing threshold was measured by 5 dB (A) step-up method.
Measurement of ambient noise
Based on the Chinese national standard for workplace noise, a noise dosimeter is used to measure the noise exposure intensity of the workplace at each post. Individual noise meters (Noise-Pro, Quest, Oconomowoc, WI, USA) were utilized in the workplace to measure noise exposure intensities for everyone for three consecutive days at ten a.m., three p.m., and five p.m.
Measurement of ambient temperature
Three measuring points were selected: dry bulb thermometer, natural wet bulb thermometer and black bulb thermometer. Three kinds of temperature are measured respectively, and the WBGT index is calculated by the following formula. Three times a day, take the average. When WBGT ≥25 °C, or WBGT-outdoor WBGT ≥2 °C, it is high temperature operation. Outdoor: WBGT = wet bulb temperature (°C) ×0.7 + black bulb temperature (°C) ×0.2 + dry bulb temperature (°C) ×0.1. Indoor: WBGT = wet bulb temperature (°C) ×0.7 + black bulb temperature (°C) ×0.3.
SNP selection
To be consistent with the purpose of the study, we focus the candidate SNPs related to the SUMO1 gene. Firstly, selected SNPs is based on the information of the Thousand Genome Project, dbSNP database and the previous literature reviews. Our criteria for identifying SNPs included: first, the minor allele frequency (MAF) in the Chinese Han population (CHB) was over 0.1. Then the linkage disequilibrium (LD) r2 was over 0.8. The SNPs locating in the gene functional regions (missense region, 3´UTR region, or 5´UTR region) or previously being reported were selected. At last, we found that rs6709162 was satisfactory, and can be used in follow-up experiments.
SNP genotyping
In this study, the target SNP was genotyped by Taqman genotyping technologies using ABI 7900HT (Applied Biosystems, Massachusetts, USA). SDS software (Applied Biosystems, v2.4) was used to analyze the results of genotyping.
Statistical analysis
All data were analyzed by SPSS 24.0 software (IBM, NYC, USA). Missing data was excluded. The measurement data such as age, duration of noise-exposed (years), noise exposure levels (dB) and high-frequency hearing threshold (dB) were expressed as mean ± SD, and the differences were analyzed by t-test. The classified variables were expressed by percentage, and the comparison was made by bilateral χ2 test. The goodness-of-fit χ2 test was performed to evaluate whether the SNP of the control group conformed to Hardy-Weinberg equilibrium. Gender, age, smoking status and drinking status were corrected to reduce bias by logistic regression model, and the odds ratio (OR) and its 95% confidence interval (CI) were estimated. The multifactor dimensionality reduction (MDR) analysis was performed to identify the possible interactions between SNP and occupational environmental exposure. Four different genetic models (codominant model, dominant model, recessive model, and allelic model) were used for sensitive analysis to find the effect of model alteration on the results. The value of P less than 0.05 was considered to be statistically significant.
Results
Demographic characteristics of the participants and Hardy-Weinberg test
In this study, 2,689 workers exposed to noise were potentially eligible and examined for eligibility. According to the exclusion criteria, (I) subjects with missing data, (II) subjects without blood collection, (III) subjects with ototoxic drug administration were excluded. Finally, a total of 1,225 workers (586 cases and 639 controls matching gender, age, smoking status, drinking status, noise working time and noise exposure intensity) were confirmed eligible, included and analyzed in the study. Table 1 showed that, there were no significant differences in general characteristics, year and intensity of noise-expose between the two groups (P>0.05). The average high-frequency hearing threshold of the NIHL case group was significantly higher (35.75±9.78 dB) than that of the control group (14.06±4.15 dB) (P<0.001). Table 2 showed the database information of the rs6709162 and the result of Hardy-Weinberg test. No subjects had missing data.
Table 1
| Variables | Cases (n=586) | Controls (n=639) | P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age (years) | 0.815a | |||||
| Mean ± SD | 40.43±6.31 | 40.36±6.21 | 0.808b | |||
| ≤35 | 142 | 24.2 | 163 | 25.5 | ||
| 35–45 | 327 | 55.8 | 345 | 54.0 | ||
| >45 | 117 | 20.0 | 131 | 20.5 | ||
| Sex | 0.611b | |||||
| Male | 542 | 92.5 | 586 | 91.7 | ||
| Female | 44 | 7.5 | 53 | 8.3 | ||
| Tobacco use | 0.411b | |||||
| Now | 337 | 57.5 | 349 | 54.6 | ||
| Ever | 12 | 2.0 | 19 | 3.0 | ||
| Never | 237 | 40.4 | 271 | 42.4 | ||
| Alcohol consumption | 0.960b | |||||
| Now | 239 | 40.8 | 257 | 40.2 | ||
| Ever | 10 | 1.7 | 12 | 1.9 | ||
| Never | 337 | 57.5 | 370 | 57.9 | ||
| Duration of noise-exposure (years) | 0.303a | |||||
| Mean ± SD | 18.54±7.60 | 18.08±7.38 | 0.535b | |||
| ≤16 | 262 | 44.7 | 297 | 46.5 | ||
| >16 | 324 | 55.3 | 342 | 53.5 | ||
| Levels of noise exposure (dB) | 0.919a | |||||
| Mean ± SD | 87.22±7.63 | 87.17±7.53 | 0.985b | |||
| ≤85 | 262 | 44.7 | 284 | 44.4 | ||
| 85–92 | 114 | 19.5 | 123 | 19.2 | ||
| >92 | 210 | 35.8 | 232 | 36.3 | ||
| High frequency hearing threshold (dB) | <0.001a | |||||
| Mean ± SD | 35.75±9.78 | 14.06±4.15 | <0.001b | |||
| ≤26 | 61 | 10.4 | 639 | 100.0 | ||
| >26 | 525 | 89.6 | 0.0 | 0.0 | ||
a, Students’ t-test; b, two-sided χ2 test.
Table 2
| SNP | Gene | Alleles | Chromosome | Functional Consequence | MAF | P for HWE | |
|---|---|---|---|---|---|---|---|
| Control | Database | ||||||
| rs6709162 | SUMO-1 | C/T | 2:202233836 | Intron variant | 0.337 | 0.341 | 0.802 |
a, data from NCBI dbSNP; b, P value of Hardy-Weinberg test.
Analysis of SNPs and NIHL risk
The logistic analysis in Table 3 showed results adjusting for gender, age, alcohol use, and tobacco use. The genotype frequencies of rs6709162 in the two groups was statistically significant different in codominant model, dominant model, recessive model, and allelic model (P<0.001, P=0.041, P<0.001, P=0.001, respectively). Workers with the rs6709162TT genotype had a relatively increased NIHL risk with the OR value of 1.92 (95% CI, 1.35–2.75) in the codominant model. The NIHL risk of workers with the TT genotype was relatively larger with an OR value of 1.81 (1.30–2.52) in the recessive model. And the NIHL risk of workers of the T allele increased in the allele model with an OR value of 1.32 (95% CI, 1.12–1.55).
Table 3
| Genetic model | Genotype | Cases | Controls | Pa | Adjusted Pb | Adjusted OR |
|||
|---|---|---|---|---|---|---|---|---|---|
| n=586 | % | n=639 | % | ||||||
| Codominant | CC | 220 | 37.5 | 276 | 43.2 | 0.001 | <0.001 | 1.00 (Ref.) | |
| CT | 264 | 45.1 | 296 | 46.3 | 1.13 (0.88–1.43) | ||||
| TT | 102 | 17.4 | 67 | 10.5 | 1.92 (1.35–2.75) | ||||
| Dominant | CC | 366 | 62.5 | 276 | 43.2 | 0.048 | 0.041 | 1.00 (Ref.) | |
| CT/TT | 220 | 37.5 | 363 | 56.8 | 1.27 (1.01–1.60) | ||||
| Recessive | CC/CT | 484 | 82.6 | 572 | 89.5 | <0.001 | <0.001 | 1.00 (Ref.) | |
| TT | 102 | 17.4 | 67 | 10.5 | 1.81 (1.30–2.52) | ||||
| Alleles | C | 704 | 60.1 | 848 | 66.4 | 0.001 | 0.001 | 1.00 (Ref.) | |
| T | 468 | 39.9 | 430 | 33.6 | 1.32 (1.12–1.55) | ||||
a, two-sided χ2 test; b, adjusted for age, gender, alcohol use and tobacco use in logistic regression model.
Stratification analysis
In the Table 4, for individuals exposed to noise levels above 92 dB (A), carrying rs6709162 TT genotype (adjusted OR =2.83, 95% CI, 1.53–5.25) in the codominant model, rs6709162 CT+TT genotype (with adjusted OR =1.47, 95% CI, 1.00–2.16) in the dominant model, rs6709162 TT genotype (with adjusted OR =2.56, 95% CI, 1.44–4.56) in the recessive model and rs6709162 T (with adjusted OR =1.84, 95% CI, 1.17–2.03) in allelic model show an increased risk for NIHL.
Table 4
| Genetic model | Group | Genotype | Expose level of noise (dB) | ||
|---|---|---|---|---|---|
| ≤85 | 85–92 | >92 | |||
| Codominant | Case | CC | 104 | 40 | 76 |
| CT | 117 | 53 | 94 | ||
| TT | 41 | 21 | 40 | ||
| Control | CC | 116 | 54 | 20 | |
| CT | 135 | 55 | 106 | ||
| TT | 33 | 14 | 106 | ||
| Pa | 0.383 | 0.203 | 0.004 | ||
| Adjusted Pb | 0.340 | 0.302 | 0.003 | ||
| Adjusted OR (95% CI)b | 1.44 (0.84–2.47) | 1.90 (0.84–4.28) | 2.83 (1.53–5.25) | ||
| Dominant | Case | CC | 104 | 40 | 76 |
| CT/TT | 158 | 74 | 134 | ||
| Control | CC | 116 | 54 | 106 | |
| CT/TT | 168 | 69 | 126 | ||
| Pa | 0.794 | 0.185 | 0.053 | ||
| Adjusted Pb | 0.698 | 0.277 | 0.049 | ||
| Adjusted OR (95% CI)b | 1.07 (0.76–1.51) | 1.35 (0.79–2.31) | 1.47 (1.00–2.16) | ||
| Recessive | Case | CC/CT | 221 | 93 | 170 |
| TT | 41 | 21 | 40 | ||
| Control | CC/CT | 251 | 109 | 212 | |
| TT | 33 | 14 | 20 | ||
| Pa | 0.143 | 0.163 | 0.001 | ||
| Adjusted Pb | 0.211 | 0.145 | 0.002 | ||
| Adjusted OR (95% CI)b | 1.45 (0.88–2.40) | 1.70 (0.80–3.61) | 2.56 (1.44–4.56) | ||
| Alleles | Case | C | 325 | 133 | 246 |
| T | 199 | 95 | 174 | ||
| Control | C | 367 | 163 | 318 | |
| T | 201 | 83 | 146 | ||
| Pa | 0.375 | 0.075 | 0.002 | ||
| Adjusted Pb | 0.313 | 0.138 | 0.002 | ||
| Adjusted OR (95% CI)b | 1.14 (0.89–1.46) | 1.33 (0.91–1.95) | 1.84 (1.17–2.03) | ||
a, two-sided χ2 test; b, adjusted for age, gender, alcohol use and tobacco use in a logistic regression model.
Analysis of gene and environment interactions
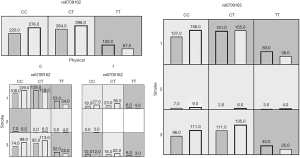
MDR software was performed to detect the interaction of rs6709162 with environmental factors. Table 5 and Figure 1 showed that, rs6709162 and smoking had a statistical interaction with P=0.0021 (OR =1.44, 95% CI, 1.14–1.81). rs6709162, smoking and physical (high temperature) also had a statistical interaction (P=0.0001, OR =1.59, 95% CI, 1.26–2.01).
Table 5
| Model | Training balanced accuracy | Testing balanced accuracy | Cross-validation consistency | P | OR (95% CI) |
|---|---|---|---|---|---|
| rs6709162 | 0.5346 | 0.5346 | 10/10 | 0.0005 | 1.80 (1.29–2.51) |
| rs6709162, smoke | 0.5435 | 0.5206 | 6/10 | 0.0021 | 1.44 (1.14–1.81) |
| rs6709162, smoke, physical | 0.555 | 0.5168 | 5/10 | 0.0001 | 1.59 (1.26–2.01) |
Discussion
NIHL has been known to be the interaction result of genetic and environmental factors, and clinical association studies have confirmed that NIHL has genetic predisposition (16,17). In this study, the relationship between SUMO-1 gene and NIHL susceptibility was discussed for the first time. It was found that exposure to high levels of noise (>92 dB) and carrying rs6709162T alleles will increase the risk of NIHL. Environmental factors such as smoking and high temperature interact with rs6709162. Related studies show that NIHL is linked to physical (high temperature) (18) environmental factors and living habits (smoking) (8), which are consistent with the results of this study.
Studies have shown that SUMO-1 belongs to the SUMO family, which can compete with ubiquitin for receptor binding sites of some substrate proteins, inhibit ubiquitin degradation of p53 protein and other substrates, and enhance the stability and transcriptional activity of wild-type p53 and other tumor suppressor genes. Long-term exposure to impulse noise can activate phosphorylated p53 and cause apoptosis of outer hair cells and Sertoli cells (19-21).
rs6709162 is located in the non-coding intron region of SUMO-1 gene. It was previously thought that only exons would be translated into proteins, while introns would be cut off during pre-mRNA processing into mature mRNA without biological function. However, with the in-depth study of gene regulatory transcription, it is found that intron is an important component of the genome, which plays a crucial role in maintaining the specific structure of chromosomes, ensuring the particular function of genes, regulating gene expression and so on (22). In post-transcriptional processing, intron regions have more mutations than exon regions. Some studies have showed that intron mutations of insulin-like growth factor 2 (IGF2), ESR (estrogen receptor), FSH β, Nodal, CPT-Iα, β-actin, β-keratin and immunoglobulin can cause changes in gene expression. Intron gene polymorphism has also been shown to be associated with numerous diseases such as aortic dissection (23), diabetes (24), and schizophrenia (25) in the studies of Ekmekci, Mazaheri and Železníková. A deletion in 19q13.32 region including SAE1 gene, which codes a SUMO-1 activating enzyme subunit, was reported by Leal on a patient with severe mental retardation, deafness, megacolon tetralogy of Fallot, cleft lip and palate, and other dysmorphic features. This case report indicates that, genome deletion in SUMO-1 region may associated with hearing loss (26).
We speculate that rs6709162 site mutation may increase the expression of SUMO-1 gene, overexpression of SUMO-1 gene and long-term exposure to high levels of noise activates p53 in hair cells and Sertoli cells of the cochlea, resulting in structural damages to hair cells and Sertoli cells, thus increasing the risk of NIHL. However, due to the lack of environmental noise exposure information such as traffic noise and music noise, measurement bias may exit.
There were several limitations to this case-control study. First, this is a retrospective case-control study, the evidence is relative weak, our findings can be validated in a prospective cohort study in the future. Second, we can conduct a multi-center study or GWAS study in a larger population to confirm this risk SNP of SUMO-1 gene. Third, future experimental research will clarify the underlying mechanism of the association between SNP and risk of NIHL.
Because of the concealment and irreversibility of NIHL, it is impossible to cure the hearing loss caused by noise. Primary prevention of NIHL is the key to reduce the incidence. On the one hand, factories should properly deduce the noise intensity and the duration of noise exposure of workers; on the other hand, they should find new ideas for noise-induced hearing prevention (27). At present, the application of noise deafness susceptibility genes in clinical diagnosis, prevention and treatment of NIHL has not been realized (28). However, with the continuous recognition and study of the susceptible genes of noise-induced deafness, it is believed that one day the susceptible individuals can be screened out before they are exposed to high noise. For workers with susceptibility genes, we should inform and guide them to develop good living habits, pay attention to personal protection in work places, and advise them to stay away from the noise in the living environment. And regular occupational health examination, once found that there is high-frequency hearing loss have been transferred from the noise job in time. The compensation of NIHL workers shall have the same rights as other employees. Therefore, studying the relationship between genes and susceptibility to NIHL and screening people susceptible to NIHL are of great significance on the terms of the prevention of NIHL and improving the living standards of NIHL workers.
Acknowledgments
Funding: This study was funded by Jiangsu Province’s Outstanding Medical Academic Leader program (CXTDA2017029).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jphe-20-114
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jphe-20-114
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe-20-114). Dr. BZ serves as an Editor-in-Chief of Journal of Public Health and Emergency from Jan 2017 to Dec 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study obtained the informed consent of all participants and was approved by the Ethics Committee of Jiangsu Center for Disease Control and Prevention [2014029].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mirza R, Kirchner DB, Dobie RA, et al. Occupational Noise-Induced Hearing Loss. J Occup Environ Med 2018;60:e498-e501. [Crossref] [PubMed]
- Kirchner DB, Evenson E, Dobie RA, et al. Occupational noise-induced hearing loss: ACOEM Task Force on Occupational Hearing Loss. J Occup Environ Med 2012;54:106-8. [Crossref] [PubMed]
- Nelson DI, Nelson RY, Concha-Barrientos M, et al. The global burden of occupational noise-induced hearing loss. Am J Ind Med 2005;48:446-58. [Crossref] [PubMed]
- Jia G. Current situation of occupational diseases in China. Modern Occupational Safety 2015:106-7.
- Hormozi M, Ansari-Moghaddam A, Mirzaei R, et al. The risk of hearing loss associated with occupational exposure to organic solvents mixture with and without concurrent noise exposure: A systematic review and meta-analysis. Int J Occup Med Environ Health 2017;30:521-35. [Crossref] [PubMed]
- Schaal NC, Slagley JM, Richburg CM, et al. Chemical-Induced Hearing Loss in Shipyard Workers. J Occup Environ Med 2018;60:e55-e62. [Crossref] [PubMed]
- Xu XR, Yang QY, Jiao J, et al. Association between variations in protocadherin 15 gene and occupational noise-induced hearing loss. Zhonghua Yu Fang Yi Xue Za Zhi 2017;51:20-6. [PubMed]
- Ding E, Guo J, Ge X, et al. Analysis of Polymorphisms Associated with Base Excision Repair in Patients Susceptible and Resistant to Noise-Induced Hearing Loss. Dis Markers 2019;2019:9327106 [Crossref] [PubMed]
- Yuan H, Deng R, Zhao X, et al. SUMO1 modification of KHSRP regulates tumorigenesis by preventing the TL-G-Rich miRNA biogenesis. Mol Cancer 2017;16:157. [Crossref] [PubMed]
- Bellail AC, Olson JJ, Hao C. SUMO1 modification stabilizes CDK6 protein and drives the cell cycle and glioblastoma progression. Nat Commun 2014;5:4234. [Crossref] [PubMed]
- Chen T, Liao XP, Wen GQ, et al. The effect of small ubiquitin-like modifier-1 modification on the formation of Lewy body-like inclusions in cytoplasm and apoptosis of HEK293 cell induced by overexpression and mutation of alpha-synuclein. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2011;28:511-6. [PubMed]
- Guo S, Zhang G, Wang Y, et al. Association between small ubiquitin-related modifier-1 gene polymorphism and non-syndromic oral clefting. Hua Xi Kou Qiang Yi Xue Za Zhi 2012;30:97-102. [PubMed]
- Ke J, Yang Y, Che Q, et al. Prostaglandin E2 (PGE2) promotes proliferation and invasion by enhancing SUMO-1 activity via EP4 receptor in endometrial cancer. Tumour Biol 2016;37:12203-11. [Crossref] [PubMed]
- Liu K, Wang X, Li D, et al. Ginkgolic Acid, a SUMO-1 Inhibitor, Inhibits the Progression of Oral Squamous Cell Carcinoma by Alleviating SUMOylation of SMAD4. Mol Ther Oncolytics 2020;16:86-99. [Crossref] [PubMed]
- Jin L, Shen K, Chen T, et al. SUMO-1 Gene Silencing Inhibits Proliferation and Promotes Apoptosis of Human Gastric Cancer SGC-7901 Cells. Cell Physiol Biochem 2017;41:987-98. [Crossref] [PubMed]
- Carvalho LCL, Marchiori LLM, Melo JJ, et al. Interleukin-1β gene polymorphism and hearing loss related to the history of occupational noise exposure in Brazilian elderly. 2013;15:160-4.
- Li H, Zhang X, Tan P, et al. Changes of cognitive function and sumo modification in hippocampus of APP/PS1 double transgenic mice. Neurological Diseases and Mental Health 2015;15.
- Yu SF, Chen GS, Jiao J, et al. A cohort study on occupational noise induced hearing loss in workers at an iron and steel plant. Zhonghua Yu Fang Yi Xue Za Zhi 2017;51:13-9. [PubMed]
- Comeron JM, Kreitman M. The correlation between intron length and recombination in drosophila. Dynamic equilibrium between mutational and selective forces. Genetics 2000;156:1175-90. [PubMed]
- Fetoni AR, Bielefeld EC, Paludetti G, et al. A putative role of p53 pathway against impulse noise induced damage as demonstrated by protection with pifithrin-alpha and a Src inhibitor. Neurosci Res 2014;81-82:30-7. [Crossref] [PubMed]
- Zhou YD, Ding DL, Zheng HL, et al. Hair cells apoptosis and the activation of P53 protein in intensive impulse noise induced cochlear lesion. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2011;46:54-8. [PubMed]
- Visser M, Palstra RJ, Kayser M. Allele-specific transcriptional regulation of IRF4 in melanocytes is mediated by chromatin looping of the intronic rs12203592 enhancer to the IRF4 promoter. Hum Mol Genet 2015;24:2649-61. [Crossref] [PubMed]
- Ekmekci A, Uluganyan M, Gungor B, et al. Association between endothelial nitric oxide synthase intron 4a/b polymorphism and aortic dissection. Turk Kardiyol Dern Ars 2014;42:55-60. [Crossref] [PubMed]
- Zeleznikova V, Vedralova M, Kotrbova-Kozak A, et al. The intron 4 polymorphism in the calcium-sensing receptor gene in diabetes mellitus and its chronic complications, diabetic nephropathy and non-diabetic renal disease. Kidney Blood Press Res 2014;39:399-407. [Crossref] [PubMed]
- Mazaheri H, Saadat M. Susceptibility to schizophrenia and insertion/deletion polymorphism in intron 3 of the XRCC4 gene. Psychiatry Res 2015;228:972-3. [Crossref] [PubMed]
- Leal T, Andrieux J, Duban-Bedu B, et al. Array-CGH detection of a de novo 0.8Mb deletion in 19q13.32 associated with mental retardation, cardiac malformation, cleft lip and palate, hearing loss and multiple dysmorphic features. Eur J Med Genet 2009;52:62-6. [Crossref] [PubMed]
- Xu L, Yin M, Zhang J. Research progress on prevention and treatment of noise-induced hearing impairment. Journal of Naval Medicine 2006;27:360-3.
- Xue X, Chen X, Xu J, et al. Research progress of susceptibility genes related to noise-induced deafness. Chinese Journal of Otology 2020;18:168-73.
Cite this article as: Zhao Q, Wu W, Li C, Yin H, Zhu B. Small ubiquitin-like modifier 1 gene associated with noise-induced hearing loss in Chinese workers. J Public Health Emerg 2020;4:33.


