
Functional BCL-2 rs2279115 Noncoding variant associated with noise-induced hearing loss in Chinese workers: a case-control study
Introduction
Noise-induced hearing loss (NIHL) is one of the most common form of severe sensorineural hearing impairment (1). At present, more than 10 million employees work in environments with excessive noise levels in China and millions of them experience varying degrees of hearing impairment (2). Both environmental and genetic factors contribute to the occurrence and severity of NIHL (3). Our previous studies illustrated that Notch, SIK3, KCNE1, and ATP2B2 genes were associated with NIHL in Chinese workers (4-8).
The BCL-2 gene encodes an integral outer mitochondrial membrane protein that blocks the apoptotic death of some cells such as lymphocytes. Studies have provided evidence that BCL-2 and the Bcl-2 pathway play crucial roles in both age-related hearing loss and NIHL. Huang et al. transfected an miR-34a mimic or miR-34a inhibitor into primary auditory cortex neurons and found a link between age-related apoptosis in auditory cortex neurons and miR-34a/Bcl-2 signaling (9). The p53 and Bcl-2 immunoreactivity was found by Xu et al. to elevate in aging hair cells, showing early signs of apoptotic changes in the nuclei, and Bcl-2 expression was increased in hair cells displaying early signs of necrosis (10). Yamashita et al. uncovered an important role of Bcl-2 family proteins in the prevention of sensory cell death following TTS (temporary threshold shift) levels of noise, and PTS (permanent threshold shift) exposure provoked the expression of Bak-associated cell death (11). Some studies have indicated that, sensorineural hearing neuron apoptosis and damage can be induced by noise exposure (12,13). FAM136A also encodes a mitochondrially localized protein. The FAM136A gene is expressed in rat neurosensorial epithelium of the crista ampullaris and a mutation in this gene has been associated with familial Meniere’s disease, a chronic disorder of the inner ear (14). L3HYPDH encodes a dehydratase that may function to degrade dietary proteins that contain trans-3-hydroxy-L-proline as well as collagen IV and other proteins. Alport syndrome (a progressive hereditary renal disease) characterized by sensorineural hearing loss and ocular abnormalities has been known as a genetic disorder (15). It may be arising from the mutations in the genes encoding alpha-3, alpha-4, and alpha-5 proteins of collagen IV or collagen IV alpha345 network (16).
No associations between mutations of these three genes and NIHL have been reported. Some studies have showed that mutations of genes can cause changes in relevant gene expression (17). Considering the vital functions of BCL-2, FAM136A, and L3HYPDH in hearing loss, we proposed that polymorphisms in the BCL-2, FAM136A, and L3HYPDH genes may be associated with genetic susceptibility to NIHL. Therefore, a case-control study was conducted to reveal the associations between functional single nucleotide polymorphisms (SNPs) in BCL-2, FAM136A, and L3HYPDH with the genetic susceptibility to NIHL in noise-exposed Chinese workers. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jphe-20-123).
Methods
Study subjects
We conducted a case-control study to explore the associations between three functional SNPs in BCL-2, FAM136A, and L3HYPDH and NIHL susceptibility. In our research, a total of 2607 workers exposed to occupational noise from two factories in the Jiangsu province of China were recruited in October 2018. Chinese occupational NIHL diagnostic criteria (GBZ49-2014) were applied to diagnose NIHL. First, all workers exposed to noise levels exceeding 85dB (A) were selected. The case and control groups were defined as follows. In the case group, the average binaural high-frequency (3,000, 4,000, and 6,000 Hz) hearing threshold was >25 dB (A), or it was ≤25 dB (A) and the worse ear’s speech frequency (500, 1,000, and 2,000 Hz) hearing threshold was >25 dB (A). The control group was defined as workers with an average binaural high-frequency hearing threshold of ≤25 dB (A) and the worse ear’s speech frequency hearing threshold was ≤25 dB (A). The subjects with missing data, ototoxic drug administration, or without blood collection were excluded. Finally, 482 cases and 482 controls with matched sex, age, smoking, drinking and noise working time were included in this study. All subjects in the two factories who met the screening criteria were included in this study. All data (questionnaire, pure-tone audiometry, measurement of ambient noise, and temperature) in this research were collected by trained investigators according to relevant guidelines and regulations. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent of all participants was obtained and the current research was approved by the Ethics Committee of Jiangsu Provincial Center for Disease Control and Prevention (2014029).
Questionnaire investigation
The questionnaire included the following parts: general demographic characteristics (e.g., gender, age, and nationality), history of diseases that may affect hearing (e.g., primary ear disease and history of ototoxic drug use), and living habits (e.g., history of smoking and drinking). In this study, smoking status was divided into two levels, smoking and no smoking. Similarly, drinking status was divided into two groups, drinking and no drinking. If a worker smoked a cigarette or drank 50 grams of alcohol (spirits and beer) a day for more than one year, they were assigned to the smoking or drinking group, respectively. Otherwise, they were assigned to the no drinking group and no smoking groups.
Pure-tone audiometry
Before the pure tone audiometry test, the subjects were instructed to avoid exposure to noise (>85 dB) for at least 12 hours. Then, 500, 1,000, 2,000, 3,000, 4,000, and 6,000 Hz pure tone air threshold tests were performed by an otorhinolaryngologist in the sound attenuation room. According to the Chinese Diagnostic Criteria of Occupational Noise-induced Hearing Loss (GBZ49-2014), the binaural hearing threshold was measured by a 5 dB (A) step-up method.
Measurement of ambient noise
According to GBZ/T 189.8-2007 “Measurement of physical factors in the workplace - Part 8: Noise”, a noise dosimeter is used to measure the noise exposure level of the workplace at each post. Individual sound pressure noise meters (Noise-Pro, Quest, Oconomowoc, WI, USA) were applied to measure the noise exposure levels for three consecutive days at ten a.m., three p.m., and five p.m.
SNP selection
Firstly, SNPs were selected based on the 1000 Genome Project data (https://www.genome.gov/27528684/1000-genomes-project), the NCBI (National Center of Biotechnology Information) dbSNP database (https://www.ncbi.nlm.nih.gov/), and a previous literature review. Our criteria for identifying SNPs included a minor allele frequency (MAF) in the Han Chinese population (CHB) of >10%. Then, the SNPs located in the gene functional regions such as missense, promoter binding, 3′UTR, and 5′UTR or previously reported to be related to human diseases were considered. In the end, rs2279115, rs3860, and rs8660 in BCL-2, FAM136A, and L3HYPDH, respectively, were found to be reported before and included.
DNA extraction and SNP genotyping
QIAcube HT and QIAamp 96 DNA QIAcube HT Kits (Qiagen, Dusseldorf, Germany) were used to extract the DNA from about 200 ul blood samples in ethylenediaminetetraacetic acid (EDTA) at −20 °C. In this study, the target SNPs in BCL-2, FAM136A, and L3HYPDH genes were genotyped by Taqman genotyping technology using an ABI 7900HT (Applied Biosystems, Massachusetts, USA). PCR primers and TaqMan probes were designed for different SNP sites on chromosome for real-time PCR amplification. The 5’- and 3’-ends of the probe were labeled with a reporter fluorescent group and a quenched fluorescent group, respectively. When PCR products were present in the solution, the probe annealed with the template to produce a substrate suitable for the activity of exonuclease. The fluorescent molecules connected with the 5’-end of the probe were cut off from the probe to destroy the pret between the two fluorescent molecules and emit fluorescence SDS v2.4 software (Applied Biosystems, Massachusetts, USA) was used to analyze the genotyping results.
Statistical analysis
All data were analyzed by SAS 9.4 software. Measurement data such as age, duration of noise-exposed years, and high-frequency hearing threshold (dB) are expressed as the mean ± SD, and the differences between the two groups were analyzed by two-sided t-test. The classified variables are expressed as percentages, and the χ2 test was used to analyze differences between the groups. The goodness-of-fit χ2 test was used to evaluate whether the SNPs in the control group conformed to Hardy-Weinberg equilibrium (HWE). Age, sex, and the duration of noise-exposed years were corrected by logistic regression model to reduce bias, and the odds ratio (OR) and 95% confidence interval (CI) were estimated. The SHEsis, a web-based platform for haplotype construction and analyses, was used to analyze haplotypes. Multifactor dimensionality reduction (MDR) analysis was applied to detect potential interactions among the SNPs. Three different genetic models (codominant model, dominant model, and recessive model) were used for sensitive analysis to find the effect of model alteration on the results. A P value of <0.05 indicated statistical significance. Missing data were not included in analysis.
Results
Demographic characteristics of the study subjects and the Hardy-Weinberg test
In this study, a total of 2,607 workers exposed to noise were potentially eligible and examined according to the inclusion and exclusion criteria. Finally, a total of 964 workers (482 cases and 482 controls matched for sex, age, smoking and drinking history, and noise exposure years) were included and analyzed in this study. As shown in Table 1, no significant differences in age, sex, tobacco use, alcohol consumption, or the number of noise-exposed years were found between the case and control groups (P>0.05). The average binaural high-frequency hearing threshold in the case group was significantly higher [37.48±11.78 dB (A)] than that in the control group [18.21±5.40 dB (A)] (P<0.001). Table 2 shows the general information of rs2279115, rs3860, and rs8660 and the Hardy-Weinberg test results. The MAF of SNPs rs2279115, rs3860, and rs8660 was ≥10%. Rs2279115 and rs8660 conformed to HWE (P>0.05). Of the participants, 466 had data missing on tobacco use and alcohol consumption.
Table 1
| Variables | Cases (n=482) | Controls (n=482) | P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age (years) | ||||||
| Mean ± SD | 46.44±6.83 | 46.40±6.78 | 0.917a | |||
| ≤35 | 37 | 7.68 | 38 | 7.88 | 0.952b | |
| 35–45 | 113 | 23.44 | 109 | 22.61 | ||
| >45 | 332 | 68.88 | 335 | 69.50 | ||
| Sex | ||||||
| Male | 443 | 91.91 | 441 | 91.49 | 0.815b | |
| Female | 39 | 8.09 | 41 | 8.51 | ||
| Tobacco use | ||||||
| No | 96 | 38.55 | 100 | 40.16 | 0.714b | |
| Yes | 153 | 61.45 | 149 | 59.84 | ||
| Alcohol consumption | ||||||
| No | 99 | 39.76 | 95 | 38.15 | 0.713b | |
| Yes | 150 | 60.24 | 154 | 61.85 | ||
| Duration of noise exposed work (years) | ||||||
| Mean ± SD | 24.86± 9.19 | 24.81±9.17 | 0.933a | |||
| ≤20 | 130 | 26.97 | 131 | 27.18 | 0.942b | |
| >20 | 352 | 73.03 | 351 | 72.82 | ||
| High frequency hearing threshold (dB) | ||||||
| Mean ± SD | 37.48±11.78 | 18.21±5.40 | <0.001a | |||
a, students’
Table 2
| Gene | SNP | Alleles | Chromosome | Functional consequence | MAF | P for HWE | |
|---|---|---|---|---|---|---|---|
| Control | Database | ||||||
| BCL-2 | rs2279115 | A/C | 18:63319604 | Noncoding region | 0.409 | 0.433 | 0.289 |
| FAM136A | rs3806 | A/G | 13:102678368 | 3’UTR | 0.405 | 0.463 | 0.010 |
| L3HYPDH | rs8660 | A/G | 14:59473009 | missense | 0.104 | 0.098 | 0.706 |
a, data from NCBI dbSNP; b, P value of Hardy-Weinberg test. SNP, single nucleotide polymorphism; MAF, minor allele frequency; HWE, Hardy-Weinberg equilibrium.
Analysis of SNPs and NIHL risk
The logistic regression analysis of the genotype frequency of BCL-2 rs2279115 in the NIHL case and control groups adjusted for age, gender, and the number of noise-exposed years showed statistical significance in the four different genetic models (codominant model, dominant model, and recessive model) (P=0.0106, 0.0074, and 0.0255, respectively) (Table 3). In the codominant model, workers with the rs2279115 CC and AC genotypes had an increased NIHL risk with an OR of 1.310. The OR in the dominant model was 1.622 for AC/CC and 1.341 for CC in the recessive model (logistic regression adjusted for age, gender, and noise exposure years). The distributions of FAM136A rs3806 and L3HYPDH rs8660 were not significantly different between the two groups.
Table 3
| Genetic models | Genotypes | Cases | Controls | Pa | Adjusted OR | |||
|---|---|---|---|---|---|---|---|---|
| n=482 | % | n=482 | % | (95% CI)b | ||||
| rs2279115 | ||||||||
| Codominant | AA | 59 | 12.24 | 89 | 18.46 | 0.0106 | 1.00 (Ref.) | |
| AC | 212 | 43.98 | 216 | 44.81 | 1.310 (1.092–1.570) | |||
| CC | 211 | 43.78 | 177 | 36.72 | ||||
| Dominant | AA | 59 | 12.24 | 89 | 18.46 | 0.0074 | 1.00 (Ref.) | |
| AC/CC | 423 | 87.76 | 393 | 81.54 | 1.622 (1.135–2.319) | |||
| Recessive | AA/AC | 271 | 56.22 | 305 | 63.28 | 0.0255 | 1.00 (Ref.) | |
| CC | 211 | 43.78 | 177 | 36.72 | 1.341 (1.035–1.737) | |||
| rs3806 | ||||||||
| Codominant | AA | 74 | 15.35 | 86 | 17.84 | 0.4205 | 1.00 (Ref.) | |
| AG | 213 | 44.19 | 218 | 45.23 | 0.888 (0.743–1.062) | |||
| GG | 195 | 40.46 | 178 | 36.93 | ||||
| Dominant | AA/AG | 287 | 59.54 | 304 | 63.07 | 0.2609 | 1.00 (Ref.) | |
| GG | 195 | 40.46 | 178 | 36.93 | 0.863 (0.665–1.119) | |||
| Recessive | AA | 74 | 15.35 | 86 | 17.84 | 0.2989 | 1.00 (Ref.) | |
| GG/AG | 408 | 84.65 | 396 | 82.16 | 0.837 (0.595–1.177) | |||
| rs8660 | ||||||||
| Codominant | AA | 393 | 81.54 | 393 | 81.54 | 0.8111 | 1.00 (Ref.) | |
| AG | 75 | 15.56 | 78 | 16.18 | 0.970 (0.740–1.272) | |||
| GG | 14 | 2.90 | 11 | 2.28 | ||||
| Dominant | AA/AG | 468 | 97.10 | 471 | 97.72 | 0.5432 | 1.00 (Ref.) | |
| GG | 14 | 2.90 | 11 | 2.28 | 0.784 (0.352–1.746) | |||
| Recessive | AA | 393 | 81.54 | 393 | 81.54 | 1.0000 | 1.00 (Ref.) | |
| GG/AG | 89 | 18.46 | 89 | 18.46 | 0.997 (0.720–1.384) | |||
a, two-sided χ2 test; b, adjusted for age, gender, noise exposure year in logistic regression model. NIHL, noise-induced hearing loss.
Stratification analysis
Table 4 shows that individuals exposed to noise for over 20 years and carrying the rs2279115 AC/CC genotype (adjusted OR =1.408, 95% CI: 1.141–1.737) in the dominant model, the rs2279115 CC genotype (adjusted OR =1.431, 95% CI: 1.059–1.934) in the recessive model, and rs2279115 AC+CC (adjusted OR =1.921, 95% CI: 1.266–2.916) in the codominant model had an increased risk for NIHL.
Table 4
| Model | Group | Genotype | Noise exposure year | |
|---|---|---|---|---|
| ≤20 | >20 | |||
| Dominant | Case | AA | 18 | 41 |
| AC/CC | 112 | 311 | ||
| Control | AA | 18 | 71 | |
| AC/CC | 113 | 280 | ||
| Pa | 0.9802 | 0.0019 | ||
| Adjusted OR (95% CI)b | 0.984 (0.485–1.995) | 1.921 (1.266–2.916) | ||
| Codominant | Case | AA | 18 | 41 |
| AC | 63 | 149 | ||
| CC | 49 | 162 | ||
| Control | AA | 18 | 71 | |
| AC | 67 | 149 | ||
| CC | 46 | 131 | ||
| Pa | 0.8985 | 0.0035 | ||
| Adjusted OR (95% CI)b | 1.054 (0.733–1.515) | 1.408 (1.141–1.737) | ||
| Recessive | Case | AA/AC | 81 | 190 |
| CC | 49 | 162 | ||
| Control | AA/AC | 85 | 220 | |
| CC | 46 | 131 | ||
| Pa | 0.6652 | 0.0193 | ||
| Adjusted OR (95% CI)b | 1.116 (0.673–1.850) | 1.431 (1.059–1.934) | ||
a, two-sided χ2 test; b, adjusted for age, sex in logistic regression model.
Haplotype analysis results
Table 5 showed the frequencies of the inferred haplotypes among the cases and controls and their association with the risk of NIHL. The alleles of the haplotypes were arrayed as rs2279115-rs3806-rs8660. Haplotypes AAA and CGA showed decreased and increased risks for NIHL with ORs of 0.80 and 1.26, respectively, compared to the groups with mixed haplotypes.
Table 5
| Haplotypesa | Case (n=964) | Control (n=964) | Pb | Adjusted OR | Global P | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | (95% CI)c | ||||
| AAA | 249.43 | 25.9 | 303.48 | 31.5 | 0.03 | 0.80 (0.65–0.98) | 0.05 | |
| AAG | 70.08 | 7.3 | 79.44 | 8.2 | 0.58 | 0.91 (0.65–1.27) | ||
| CGA | 574.99 | 59.6 | 547.34 | 56.8 | 0.02 | 1.26 (1.04–1.52) | ||
| Othersd | 69.5 | 7.2 | 33.74 | 3.5 | 1.00 (Ref.) | |||
a, the alleles of haplotypes were arrayed as rs2279115-rs3806-rs8660; b, two-sided χ2 test; c, generated by permutation test with 1,000 times of simulation; d, haplotypes with a frequency <0.05 were pooled into the others group. NIHL, noise-induced hearing loss
Gene and environment interaction analysis
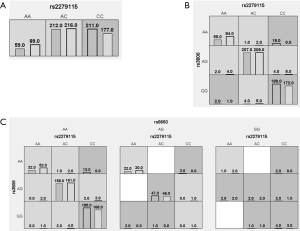
MDR v3.0.2 software was used to detect the potential interactions of BCL-2 rs2279115, FAM136A rs3806, and L3HYPDH rs8660. Table 6 and Figure 1 shows that BCL-2 rs2279115, FAM136A rs3806, and L3HYPDH rs8660 had a statistically significant interaction with increased NIHL risk (P=0.0083, OR =1.41, 95% CI: 1.09–1.81).
Table 6
| Model | Training balanced accuracy | Testing balanced accuracy | Cross-validation consistency | P | OR (95% CI) |
|---|---|---|---|---|---|
| rs2279115 | 0.5358 | 0.5218 | 10/10 | 0.0255 | 1.34 (1.04–1.74) |
| rs2279115*rs3806 | 0.5392 | 0.5104 | 6/10 | 0.0178 | 1.37 (1.06–1.77) |
| rs2279115*rs3806*rs8660 | 0.5441 | 0.5104 | 10/10 | 0.0083 | 1.41 (1.09–1.81) |
SNP, single nucleotide polymorphism; MDR, multifactor dimensionality reduction.
Discussion
In the current study, the genotype frequency of BCL-2 rs2279115 was statistically significant in the NIHL case group compared to the control group in the three different genetic models (codominant model, dominant model, and recessive model). Also, haplotype CGA (rs2279115-rs3806-rs8660) was associated with an increased risk for NIHL. Interaction analysis indicated that BCL-2 rs2279115, FAM136A rs3806, and L3HYPDH rs8660 had statistically significant interactions with increased NIHL risk. Our results provide evidence that BCL-2 polymorphism was associated with NIHL risk in Chinese workers.
The BCL-2 gene has been associated with numerous diseases (18-20). Ahmed et al. evaluated the association of BCL-2 gene polymorphism (rs2279115) and hepatitis C virus (HCV)-related hepatocellular carcinoma (HCC) susceptibility in 270 individuals and uncovered an association with a genetic polymorphism in BCL-2 (rs2279115) with susceptibility to HCV-related hepatocellular carcinoma (21). The association between the BCL-2-938C>A (rs2279115) genotype and prostate cancer outcome was studied by Renner et al. The study reported that the homozygous BCL-2-938 CC genotype was associated with overall survival in prostate cancer patients (22).
No associations between NIHL or hearing loss and BCL-2 gene polymorphism have been reported. However, some molecular studies have provided evidence that BCL-2 and the Bcl-2 pathway play crucial roles in both age-related hearing loss and NIHL. Huang et al. found a link between age-related apoptosis in auditory cortex neurons and miR-34a/Bcl-2 signaling (9). The expression of immunoreactive p53 and Bcl-2 was increased in aging hair cells showing early signs of apoptotic changes in the nuclei and Bcl-2 expression was increased in hair cells displaying early signs of necrosis (10). Yamashita et al. reported an important role of the Bcl-2 family proteins in the prevention of sensory cell death following TTS noise levels, and PTS exposure provoked the expression of Bak-associated cell death (11). BCL-2 rs2279115 is a functional genetic variant located in the inhibitory P2 promoter region. Also, the BCL-2 rs2279115A allele may have an interaction with TP53, which will eventually lead to decreases in Bcl-2 expression levels (23).
BCL-2 rs2279115 was also found to be associated with noised-induced hearing loss. A possible reason may be the function of rs2279115 to inhibit the P2 promoter and subsequently alter Bcl-2 expression levels. Bcl-2 has been shown to induce sensory cell death in animals. There were several limitations to this case-control study. First, all NIHL cases were recruited from two factories in this retrospective study, which may lead to selection bias. Then, this is a retrospective case-control study. The evidence of association of BCL-2 polymorphism and NIHL is relative weak. As a result, our findings need to be validated in a prospective study in the future. Second, a larger population of multi-centric study can be conducted to confirm this risk SNP of BCL-2 gene. Third, future animal or cell experimental research will clarify the underlying mechanism of the association between BCL-2 SNP and risk of NIHL. In the end, BCL-2 rs2279115 may be a potential genetic biomarker for NIHL susceptibility.
Acknowledgments
Funding: This study was funded by Jiangsu Province’s Outstanding Medical Academic Leader program (CXTDA2017029).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jphe-20-123
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jphe-20-123
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe-20-123). Dr. BZ serves as an Editors-in-Chief of Journal of Public Health and Emergency from Jan. 2017 to Dec. 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent of all participants was obtained and the current research was approved by the Ethics Committee of Jiangsu Provincial Center for Disease Control and Prevention (2014029).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Le TN, Straatman LV, Lea J, et al. Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg 2017;46:41. [Crossref] [PubMed]
- Zhang X, Wang Z, Li T. The current status of occupational health in China. Environ Health Prev Med 2010;15:263-70. [Crossref] [PubMed]
- Mirza R, Kirchner DB, Dobie RA, et al. Occupational Noise-Induced Hearing Loss. J Occup Environ Med 2018;60:e498-501. [Crossref] [PubMed]
- Ding E, Guo J, Ge X, et al. Analysis of Polymorphisms Associated with Base Excision Repair in Patients Susceptible and Resistant to Noise-Induced Hearing Loss. Dis Markers 2019;2019:9327106 [Crossref] [PubMed]
- Ding E, Liu J, Shen H, et al. Notch polymorphisms associated with sensitivity of noise induced hearing loss among Chinese textile factory workers. BMC Med Genet 2018;19:168. [Crossref] [PubMed]
- Ding E, Wang H, Han L, et al. Variations in the potassium voltage-gated channel subfamily E regulatory subunit 1 gene associated with noise-induced hearing loss in the Chinese population. Environ Sci Pollut Res Int 2020;27:18822-30. [Crossref] [PubMed]
- Yin H, Guo J, Ding E, et al. Salt-Inducible Kinase 3 Haplotypes Associated with Noise-Induced Hearing Loss in Chinese Workers. Audiol Neurootol 2020;25:200-8. [Crossref] [PubMed]
- Zhang S, Ding E, Yin H, et al. Research and Discussion on the Relationships between Noise-Induced Hearing Loss and ATP2B2 Gene Polymorphism. Int J Genomics 2019;2019:5048943 [Crossref] [PubMed]
- Huang Q, Ou Y, Xiong H, et al. The miR-34a/Bcl-2 Pathway Contributes to Auditory Cortex Neuron Apoptosis in Age-Related Hearing Loss. Audiol Neurootol 2017;22:96-103. [Crossref] [PubMed]
- Xu Y, Yang WP, Hu BH, et al. Involvement of p53 and Bcl-2 in sensory cell degeneration in aging rat cochleae. Acta Otolaryngol 2017;137:572-80. [Crossref] [PubMed]
- Yamashita D, Minami SB, Kanzaki S, et al. Bcl-2 genes regulate noise-induced hearing loss. J Neurosci Res 2008;86:920-8. [Crossref] [PubMed]
- Fröhlich F, Gröschel M, Strübing I, et al. Apoptosis in the cochlear nucleus and inferior colliculus upon repeated noise exposure. Noise Health 2018;20:223-31. [Crossref] [PubMed]
- Basta D, Gröschel M, Strübing I, et al. Near-infrared-light pre-treatment attenuates noise-induced hearing loss in mice. PeerJ 2020;8:e9384 [Crossref] [PubMed]
- Requena T, Cabrera S, Martin-Sierra C, et al. Identification of two novel mutations in FAM136A and DTNA genes in autosomal-dominant familial Meniere’s disease. Hum Mol Genet 2015;24:1119-26. [Crossref] [PubMed]
- Kamiyoshi N, Nozu K, Fu XJ, et al. Genetic, Clinical, and Pathologic Backgrounds of Patients with Autosomal Dominant Alport Syndrome. Clin J Am Soc Nephrol 2016;11:1441-9. [Crossref] [PubMed]
- Savige J, Sheth S, Leys A, et al. Ocular features in Alport syndrome: pathogenesis and clinical significance. Clin J Am Soc Nephrol 2015;10:703-9. [Crossref] [PubMed]
- Jia P, Zhao Z. Impacts of somatic mutations on gene expression: an association perspective. Brief Bioinform 2017;18:413-25. [PubMed]
- Qiu XG, Chen YD, Yuan J, et al. Functional BCL-2 rs2279115 Promoter Noncoding Variant Contributes to Glioma Predisposition, Especially in Males. DNA Cell Biol 2019;38:85-90. [Crossref] [PubMed]
- Mou X, Li T, Wang J, et al. Genetic Variation of BCL2 (rs2279115), NEIL2 (rs804270), LTA (rs909253), PSCA (rs2294008) and PLCE1 (rs3765524, rs10509670) Genes and Their Correlation to Gastric Cancer Risk Based on Universal Tagged Arrays and Fe3O4 Magnetic Nanoparticles. J Biomed Nanotechnol 2015;11:2057-66. [Crossref] [PubMed]
- Searle CJ, Brock IW, Cross SS, et al. A BCL2 promoter polymorphism rs2279115 is not associated with BCL2 protein expression or patient survival in breast cancer patients. Springerplus 2012;1:38. [Crossref] [PubMed]
- Ahmed HS, Wahab EA, Elhady HA, et al. Association of genetic polymorphism of BCL-2 (rs2279115) with susceptibility to HCV-related hepatocellular carcinoma. Immunol Res 2020;68:189-97. [Crossref] [PubMed]
- Renner W, Langsenlehner U, Krenn-Pilko S, et al. BCL2 genotypes and prostate cancer survival. Strahlenther Onkol 2017;193:466-71. [Crossref] [PubMed]
- Chen K, Hu Z, Wang LE, et al. Single-nucleotide polymorphisms at the TP53-binding or responsive promoter regions of BAX and BCL2 genes and risk of squamous cell carcinoma of the head and neck. Carcinogenesis 2007;28:2008-12. [Crossref] [PubMed]
Cite this article as: Zhu H, Chen H, Ying H, Zhu B. Functional BCL-2 rs2279115 Noncoding variant associated with noise-induced hearing loss in Chinese workers: a case-control study. J Public Health Emerg 2020;4:32.


