
Effectiveness of the inactivated enterovirus 71 vaccine in children aged 6–35 months: protocol for a multicentre, case-control phase IV clinical trial
Introduction
Hand foot and mouth disease (HFMD) is a common infectious disease caused by various human enteroviruses in children younger than 5 years (1). Enterovirus A 71 (EV-A71) and Coxsackievirus A 16 (CV-A16) are the two major pathogens responsible for worldwide HFMD epidemic, and increasing cases of HFMD due to other enterovirus such as CV-A6 and CV-A10 have been reported recently (2-4). Severe neurological and systemic complications of HFMD in young children are often associated with EV-A71 (5), which makes EV-A71 epidemic a serious public health problem in the Western Pacific region in the past decades. China is the most affected country, in where at least 13 million HFMDs and more than 3,000 deaths have been reported from 2008 to 2015 (6). So the rapid development of EV-A71 vaccines is motivated by the overwhelming public health need and strong market demand in China.
By March 2013, three manufactures in mainland China, including Vgioo Biological Co., Ltd (Vgioo), Sinovac Bitech Co., Ltd (Sinovac) and Chinese Academy of Medical Sciences (CAMS) have completed pivotal efficacy trials of inactivated EV-A71 vaccines in more than 30,000 infants and children (7-9). The results showed that EV-A71 vaccines had good safety and efficacy profile in infants and children, which can provide over 90% protective efficacy against EV-A71 associated HFMD and vaccine efficacy of 100% for EV-A71 associated hospitalization and severe cases. Since December 2015, inactivated EV-A71 vaccines have already be licensed by China Food and Drug Administration (CFDA) (10), and introduced for the routine childhood immunization with self-paying in mainland China.
Nonetheless, it is not well known that the effectiveness of EV-A71 vaccines under ‘real world’ conditions, in which the population vaccinated, immunization implementation and epidemic pattern of HFMD are different from that in clinical trial settings. It is important to evaluate effectiveness of licensed vaccines for the policy decisions on vaccine introduction and the optimization of the vaccine program implementation (11). The case-control method is commonly used to estimate effectiveness after a vaccine has been implemented in a public health system, include Haemophilus Influenzae type B (Hib) (12-14), Streptococcus pneumoniae (15-18), influenza (19-23), rotavirus (24-29) and cholera (30-33). The results of case-control vaccine effectiveness studies can complement and extend the data generated by clinical trials.
Here, we reported the protocol of the phase IV clinical trial for the inactivated EV-A71 vaccine. In this trial, we will employ a case-control study with two groups of controls—test-negative control and community control—to assess the effectiveness of EV-A71 vaccination in children aged 6–35 months of mainland China. Our primary objective is to assess the effectiveness of EV-A71 vaccine against EV-A71-associated HFMD. Secondary objectives are to assess: (I) the vaccine effectiveness for EV-A71-associated hospitalization and severe cases; (II) the pathogen spectrum of HFMD under different vaccine coverage of inactivated EV-A71 vaccine.
Methods
Study design
This study is a multicentre, phase IV, case-control study of the inactivated EV-A71 vaccine at ten sites in China (Jiawang district, Wujing district, Pei county, Xiangshui country, Binhai country, Haimen city, Qidong city and Gangyu district in Jiangsu province, as well as Qianjiang city and Gucheng county in Hubei province), in September 2019.
Before the case-control study is implemented, we need to increase the vaccine coverage rate to 20–40% by vaccination program. After reaching the predetermined vaccine coverage, we will establish the hospital-based active surveillance of HFMDs at study sites, and employ a case-control study to determine the vaccine effectiveness against EV-A71-associated HFMD by assessing vaccine status of patients with HFMD cases who tested positive for EV-A71 with those who tested negative and with community controls.
The protocol of this study has been approved by the Ethics Committees of Jiangsu and Hubei Provincial Center of Disease Control and Prevention. Written informed consent will be obtained from parents/guardians before children participate the study. The trial was registered with a ClinicalTrials.gov Number: NCT03582761.
Vaccination program
In general, a case-control study has the most power when the vaccine coverage is between 20% and 80% (11,34), so we will implement vaccination program to achieve a vaccine coverage of 20–40% through public health education of EV-A71 vaccine. In the vaccination program, healthy children aged 6–35 months will be recruited following the voluntary and self-paying of the parents and the description of inactivated EV-A71 vaccine (Table 1). Written informed consent will be obtained from parents before being vaccinated.
Table 1
| Inclusion criteria |
| 1. Healthy subjects aged from 6 to 35 months old |
| 2. General good health as established by medical history and physical examination |
| 3. Subjects’ guardians who allow to comply with the requirements of the protocol |
| 4. Subjects’ guardians are able to understand and sign the informed consent |
| Exclusion criteria |
| 1. Subject who has a medical history of hand, foot and mouth disease or had received the EV-A71 vaccine |
| 2. Subject who is known to be allergic to ingredient of the EV-A71 vaccine (Vero Cell) |
| 3. Patients with fever, acute disease or acute onset of chronic disease |
| 4. Patients with serious chronic illness or allergic physique |
| 5. Patients with thrombocytopenia or hemorrhagic disease |
| 6. Patients undergoing immunosuppressive therapy or immunodeficiency |
| 7. Patients with uncontrolled epilepsy and other progressive neurological diseases, such as Guillain-Barre syndrome |
Inactivated alum-adjuvanted EV-A71 vaccine (Vero cell) was manufactured by Wuhan Institute of Biological Products Co., Ltd (Drug registration approval number: 2016S00596). Vaccine is administered intramuscularly to the anterolateral side of the thigh (for children aged 6–11 months) or the deltoid muscle (those aged 12–35 months) at day 0 and 28. Vaccination will be recorded in electronic immunization records (EIRs) and the immunization cards to calculate the vaccine coverage of inactivated EV-A71 vaccine.
Surveillance of HFMD
Since 2008, hand foot and mouth disease was made statutorily notifiable in mainland China. All probable HFMD cases need to be referred to sentinel hospitals of each counties or districts, which report clinically defined HFMD cases to the electronic National Notifiable Infectious Disease Reporting Information System (NNIDRIS).
On the basis of NNIDRIS, we will establish the hospital-based active surveillance of HFMD at study sites and recruit patients aged 6–47 months who present to the hospital with HFMD defined by the clinicians. A clinically defined case of HFMD is defined as a patient with papular or vesicular rash on the hands, feet, mouth or buttocks, with or with fever. Patients with HFMD are classified as severe if they had any neurological complications (aseptic meningitis, encephalitis, encephalomyelitis, acute flaccid paralysis, or autonomic nervous system dysregulation), or cardiopulmonary complications (pulmonary oedema, pulmonary haemorrhage, or cardiorespiratory failure), or both. Otherwise, patients are categorised as mild cases (35).
After determining eligibility and obtaining informed parental consent, throat swabs and anal swabs/stool specimens will be collected from all eligible patients for testing of human enteroviruses tests by real-time revers-transcriptase polymerase chain reaction (RT-PCR).
Definition and recruitment of case and control
Cases are defined as eligible patients who tested positive for enterovirus 71. For each case, we will select two groups of control children, including hospital test-negative control and community control. In the test-negative design, clinically defined HFMD cases with universal enterovirus positive and EV-A71 negative will be eligible for enrolment as unmatched test-negative controls (Figure 1).
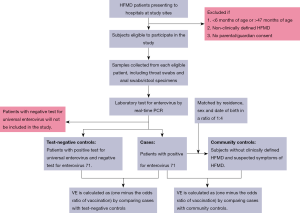
In the community case-control design, we will collect the information of children aged 6–37 months in the study sites to select eligible controls, including residence, sex and date of birth from the immunization information system. For each laboratory confirmed case of EV-A71 associated HFMD, we will select 4 community controls without clinically defined HFMD and suspected symptoms of HFMD through a random method, who are matched to cases by residence, sex and date of birth (Figure 1). Detailed inclusion and exclusion criteria for cases and controls are shown in Table 2.
Table 2
| Groups | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Cases | Aged from 6 to 47 months | Absence of verifiable EV-A71 vaccination history |
| Clinically defined HFMD | A history of HFMD caused by EV-A71 or unknown HFMD related pathogen | |
| The participants’ parents agree and sign the informed consent | Receiving the first dose of EV-A71 vaccine less than 28 days before illness onset | |
| 4. Detected specimens are EV-A71 positive by real-time RT-PCR | The place of residence is not included in the study areas | |
| Test-negative controls | Aged from 6 to 47 months | Absence of verifiable EV-A71 vaccination history |
| Clinically defined HFMD | 2. A history of HFMD caused by EV-A71 or unknown HFMD related pathogen | |
| The participants’ parents agree and sign the informed consent | 3. Receiving the first dose of EV-A71 vaccine less than 28 days before enrollment | |
| Detected specimens are positive for universal enterovirus and EV-A71 negative by real-time RT-PCR | 4. The place of residence is not included in the study areas | |
| Community controls | Aged from 6 to 47 months | 1. Absence of verifiable EV-A71 vaccination history |
| No Clinically defined HFMD, and no fever, suspected symptoms of HFMD | 2. A history of HFMD caused by EV-A71 or unknown HFMD related pathogen | |
| Near the residence of the case (the same village or adjacent village) | 3. Receiving the first dose of EV-A71 vaccine less than 28 days before enrollment | |
| The same gender as the matched case | 4. The place of residence is not included in the study areas | |
| The age is similar to that of matched case (30 days older or younger for infants <12 months, and 3 months older or younger for children ≥12 months) |
Laboratory methods
At enrollment, study staff will obtain combined throat swabs and anal swabs/stool specimens from eligible HFMD cases. Specimens will be stored at 4 °C prior to transfer to the laboratory of county/district Centers for Disease Control and Prevention (CDC) daily, where specimens are maintained at −70 °C and taken for enteroviruses detection within 24 h.
Specimens will be tested for human enteroviruses by using the real-time RT-PCR with viral RNA diagnostic kits according to the manufacturer’s instructions. The real-time RT-PCR is performed to detect the presence of the universal sequence of enterovirus and the specific sequences of EV-A71, CA-16, CA-10 and CA-6. Participants who tested positive for universal enteroviruses and EV-A71 are defined as cases, and participants who tested positive for universal enteroviruses and negative for EV-A71 are defined as test-negative controls.
Additionally, a subset of 10% of EV-A71 positive specimens will be sent to the laboratory of provincial CDC for genetic identity by sequencing VP1 gene. Reference sequence will be downloaded from GenBank (US National Center for Biotechnology Information, NCBI) and subjected to phylogenetic analysis together with our isolated. We will use MEGA 5.0 software to align the entire VP1 nucleotide sequences of the EV-A71 and conduct phylogenetic trees.
Data collection
Before the case-control study is implemented, it is needed to report monthly vaccination coverage of each township until to reach the predetermined level of 20–40%. Parents of eligible patients will be interviewed face-to-face by study staff during the medicine visit, and similarly, that of community controls will be interviewed at their homes. After determining eligibility and provision of informed parental consent, we will obtain information on socio-demographics, prematurity and parturition at birth, birth weight, history of breast feeding, medical history, history of HFMDs and vaccination history, which will be recorded in the standardized questionnaire. For medically-attended HFMD diagnosed by the clinicians, we also gather information clinical characteristics, treatment and course of illness from the parents and hospital information system (HIS). To avoid the difference of recall bias between cases and controls, the community controls will be included within 72 h of identifying the corresponding case.
Vaccination history will be defined from combination of electronic immunization records (EIRs) and parent-reported vaccination card, including EV-A71 vaccine and other routine vaccines given at the same ages. A photocopy of the vaccination record will be obtained for cases and controls. Study staff review the EIRs for all participants and the immunization card from the parent to determine receipt of EV-A71 vaccination and extract data from EIRs and immunization cards including vaccine manufacture and data of vaccination. Subjects will be considered vaccinated with the respective number dose (1 or 2) if the most recent dose of EV-A71 vaccine is administered 28 or more days before illness onset or at enrollment for controls.
Sample size
We calculated the required sample size with PASS (version 15.0) with the assumption of 80% vaccine effectiveness, vaccine coverage of 20%, and intracluster correlation coefficient within the matched groupings of 0.2 (36). In the test-negative design, EV-A71-associated HFMD is estimated to account for one third of all clinically defined HFMD cases and a sample size of 105 cases would provide a 90% statistical power to show vaccine effectiveness with assumption of exclusion rate of 20% and nonresponse rate of 15% for eligible patients. In the community case control design (1:4 case-to-control ratio), approximately 88 cases would be sufficient for assessing the vaccine effectiveness.
According to the results of efficacy trial for the inactivated EV-A71 vaccine, EV-A71-associated HFMD incidence density is estimated 2 cases per 1,000 person-years under 20–40% vaccine coverage (7). Administration of 40,000 children will achieve vaccine coverage between 20% and 40% in 100,000–200,000 target population, and it is estimated that approximately 200 EV-A71-associated HFMD cases during the study period, which would allow us to meet our desired sample size.
Statistical analysis
We first conduct bivariate analyses to examine for differences in demographic, clinical characteristics and vaccination history between cases and respective controls. Differences are examined using t test or Wilcoxon rank-sum test for continuous covariates and χ2 test for categorical covariates. Logistic regression is performed to calculate the odds ratio (OR) of vaccination in cases vs. each of the control groups, and vaccine effectiveness is calculated as (1 − odds ratio) ×100. The 95% confidence intervals for vaccine effectiveness is calculated as (1 − CIOR) ×100, where CIOR is the confidence interval of the odds ratio estimates. Crude and adjusted odds ratios will be calculated and adjusted models include variables for sex, age, prematurity and parturition at birth, birth weight, history of breast feeding, number of days between illness onset and specimen collection, calendar month.
Additionally, we estimate the vaccine effectiveness for severe HFMD caused by EV-A71 and EV-A71 associated hospitalization. Stratified effectiveness estimates are calculated by age category (age of 6–23 and 24–47months) and by dose (1 and 2 doses). For all estimates, P values of <0.05 are considered statistically significant. Statistically analyses are conducted using SAS statistical software (version 9.3).
Discussion
This is the first evaluation of the performance of EV-A71 vaccines in the context of real-world immunization programs using case-control methodology with community controls and test-negative controls, which will play an important role in guiding policy decisions and sustained use. Despite being widely used to evaluate vaccine effectiveness, the case-control methodology is susceptible to bias and confounding. Therefore, case-control vaccine effectiveness studies require rigorous planning and implementation to provide valid and reliable results.
Choosing the appropriate controls is one of the most important factors in minimizing bias in a case-control study design. Community controls are generally preferred because they are likely to be most representative of the source population giving rise to the cases (34), however, potential confounding by health care-seeking behaviors needs to be considered in case-control studies based on hospital surveillance. In general, the probability of visiting a hospital is greater in vaccinated than in unvaccinated individuals. Thus, the likelihood of there being more vaccinated individuals among cases recruited from hospitals tends to be greater than from the general population, which results in a biased underestimate of vaccine effectiveness.
In the last decade, the test-negative design has on occasion been adopted to evaluate vaccine effectiveness (11). This method assumes the vaccine being evaluated has no effect on the incidence of test-negative cases who will serve as controls. For EV-A71 vaccine, this assumption may be valid, since it showed no cross protection against HFMD caused by other enterovirus (7,8), moreover, the low vaccine coverage is not sufficient to produce selection pressure against other enterovirus. A major advantage of this design is to avoid bias due to differential healthcare-seeking behaviors between cases and controls (37,38). In this study, cases and test-negative controls can be selected from patients who visit the same hospital due to HFMD. Hence, cases and test-negative controls have similar healthcare-seeking behavior for HFMD cases. Furthermore, the test-negative design has a great potential to save time and resource relative to community controls. Also, it reduces differential recall bias of the exposure and vaccination history because case-control status is not known at the time of recruitment (11). However, test-negative controls would not avoid selection bias for receiving vaccination due to differences in healthcare-seeking behaviors or associated factors, then the results of a test-negative study may not be generalizable to the general population (39). To avoid bias from selection of controls, we use two groups of controls including community controls and test-negative controls. Additionally, we define controls as HFMD patients with positive for universal enterovirus and EV-A71 negative to avoid the misclassification of cases as control among HFMD patients with negative enterovirus due to the poor quality of samples and others. Vaccination status is the primary exposure of interest for case-control vaccine effectiveness studies, but accurate and complete ascertainment of vaccination is challenging. In this study, we will determine EV-A71 vaccination history by combining with electronic immunization records (EIRs) and parent-reported vaccination card, to minimize potential bias due to misclassification of vaccination.
Our study has some limitations. First, cases are derived from hospital-based HFMD surveillance and not from population-based surveillance, which may not be representative of all HFMD cases in the target population due to medical care-seeking behaviors. In addition, hospital-based surveillance does not allow calculation of incidence rates of EV-A71 associated HFMD. Second, only children who are admitted with HFMD will be included in the surveillance, and we cannot assess the performance of EV-A71 vaccine against other diseases induced by EV-A71 in the context of real world. Third, Jiangsu and Hubei are the central and southern provinces of China. Our results might not be representative of western and northern provinces of China with different economy and epidemic pattern. Finally, our case-control design determines only the direct protection provided by EV-A71 vaccine. The total benefit of vaccination is probably greater than that provided by direct protection as population vaccine coverage increases. Nevertheless, we will perform an exploratory analysis to evaluate the correlation between EV-A71 vaccine coverage and protective effectiveness and demonstrate the indirect effectiveness of EV-A71 vaccine.
In summary, through our attempt to address potential bias and confoundings, including identification of cases, selection of two groups of controls, the determination of vaccination status with combination of EIRs and vaccination card, uniform standard for recruitment and data collection, adjusted analysis for potential confounding factors, the findings of this study will provide valid and reliable knowledge on the performance of EV-A71 vaccines in the real-world context.
Acknowledgments
Funding: This work is supported by a grant from Wuhan Institute of Biological Products Co., Ltd (Project Number: JSCVT049).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2019.11.02). FZ serves as an Deputy Editor-in-Chief of Journal of Public Health and Emergency from January 2017 to December 2022. The other authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study has been approved by Ethics Committees of Jiangsu and Hubei Provincial Center of Disease Control and Prevention (JSJK2018-A028-02). Written informed consent will be obtained from parents/guardians before children participate the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xing W, Liao Q, Viboud C, et al. Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect Dis 2014;14:308-18. [Crossref] [PubMed]
- Lu QB, Zhang XA, Wo Y, et al. Circulation of Coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One 2012;7:e52073 [Crossref] [PubMed]
- Bian L, Wang Y, Yao X, et al. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther 2015;13:1061-71. [Crossref] [PubMed]
- Bian L, Gao F, Mao Q, et al. Hand, foot, and mouth disease associated with coxsackievirus A10: more serious than it seems. Expert Rev Anti Infect Ther 2019;17:233-42. [Crossref] [PubMed]
- McMinn PC. Enterovirus vaccines for an emerging cause of brain-stem encephalitis. N Engl J Med 2014;370:792-4. [Crossref] [PubMed]
- Huang J, Liao Q, Ooi MH, et al. Epidemiology of Recurrent Hand, Foot and Mouth Disease, China, 2008-2015. Emerg Infect Dis 2018;24: [Crossref] [PubMed]
- Zhu FC, Meng FY, Li JX, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013;381:2024-32. [Crossref] [PubMed]
- Zhu F, Xu W, Xia J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med 2014;370:818-28. [Crossref] [PubMed]
- Li R, Liu L, Mo Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 2014;370:829-37. [Crossref] [PubMed]
- Mao QY, Wang Y, Bian L, et al. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev Vaccines 2016;15:599-606. [Crossref] [PubMed]
- Verani JR, Baqui AH, Broome CV, et al. Case-control vaccine effectiveness studies: Preparation, design, and enrollment of cases and controls. Vaccine 2017;35:3295-302. [Crossref] [PubMed]
- de Andrade AL, Andrade JG, Martelli CM, et al. Effectiveness of Haemophilus influenzae b conjugate vaccine on childhood pneumonia: a case-control study in Brazil. Int J Epidemiol 2004;33:173-81. [Crossref] [PubMed]
- Baqui AH, El Arifeen S, Saha SK, et al. Effectiveness of Haemophilus influenzae type B conjugate vaccine on prevention of pneumonia and meningitis in Bangladeshi children: a case-control study. Pediatr Infect Dis J 2007;26:565-71. [Crossref] [PubMed]
- Khowaja AR, Mohiuddin S, Cohen AL, et al. Effectiveness of Haemophilus influenzae type b conjugate vaccine on radiologically-confirmed pneumonia in young children in Pakistan. J Pediatr 2013;163:S79-S85. [Crossref] [PubMed]
- Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006;368:1495-502. [Crossref] [PubMed]
- Barricarte A, Castilla J, Gil-Setas A, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine: a population-based case-control study. Clin Infect Dis 2007;44:1436-41. [Crossref] [PubMed]
- Domingues CM, Verani JR, Montenegro Renoiner EI, et al. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med 2014;2:464-71. [Crossref] [PubMed]
- Moore MR, Link-Gelles R, Schaffner W, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med 2016;4:399-406. [Crossref] [PubMed]
- McLean HQ, Thompson MG, Sundaram ME, et al. Influenza vaccine effectiveness in the United States during 2012-2013: variable protection by age and virus type. J Infect Dis 2015;211:1529-40. [Crossref] [PubMed]
- Jackson ML, Chung JR, Jackson LA, et al. Influenza Vaccine Effectiveness in the United States during the 2015-2016 Season. N Engl J Med 2017;377:534-43. [Crossref] [PubMed]
- Jefferson T, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2018;2:CD004879 [PubMed]
- Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016;16:942-51. [Crossref] [PubMed]
- Darvishian M, van den Heuvel ER, Bissielo A, et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: an individual participant data meta-analysis of test-negative design case-control studies. Lancet Respir Med 2017;5:200-11. [Crossref] [PubMed]
- Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 2009;301:2243-51. [Crossref] [PubMed]
- Snelling TL, Andrews RM, Kirkwood CD, et al. Case-control evaluation of the effectiveness of the G1P[8] human rotavirus vaccine during an outbreak of rotavirus G2P[4] infection in central Australia. Clin Infect Dis 2011;52:191-9. [Crossref] [PubMed]
- Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 2010;125:e199-207. [Crossref] [PubMed]
- de Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ 2010;340:c2825. [Crossref] [PubMed]
- Araki K, Hara M, Shimanoe C, et al. Case-Control Study of Rotavirus Vaccine Effectiveness Compared to Test-Negative Controls or Hospital Controls. J Epidemiol 2019;29:282-7. [Crossref] [PubMed]
- Tate JE, Patel MM, Cortese MM, et al. Use of Patients With Diarrhea Who Test Negative for Rotavirus as Controls to Estimate Rotavirus Vaccine Effectiveness Through Case-Control Studies. Clin Infect Dis 2016;62:S106-14. [Crossref] [PubMed]
- Luquero FJ, Grout L, Ciglenecki I, et al. Use of Vibrio cholerae vaccine in an outbreak in Guinea. N Engl J Med 2014;370:2111-20. [Crossref] [PubMed]
- Ivers LC, Hilaire IJ, Teng JE, et al. Effectiveness of reactive oral cholera vaccination in rural Haiti: a case-control study and bias-indicator analysis. Lancet Glob Health 2015;3:e162-8. [Crossref] [PubMed]
- Franke MF, Jerome JG, Matias WR, et al. Comparison of two control groups for estimation of oral cholera vaccine effectiveness using a case-control study design. Vaccine 2017;35:5819-27. [Crossref] [PubMed]
- Lucas ME, Deen JL, von Seidlein L, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med 2005;352:757-67. [Crossref] [PubMed]
- World Health Organization. Measuring impact of Streptococcus pneumoniae and Haemophilus influenzae type b conjugate vaccination, 2012. Available online: https://apps.who.int/iris/bitstream/handle/10665/75835/WHO_IVB_12.08_eng.pdf
- National Health Commission of the People’s Republic of China. Guidelines for the diagnosis and treatment of hand foot and mouth disease, 2018. Available online: http://www.nhc.gov.cn/wjw/gfxwjj/list_21.shtml.
- Bar-Zeev N, Kapanda L, Tate JE, et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis 2015;15:422-8. [Crossref] [PubMed]
- Sullivan SG, Tchetgen EJ, Cowling BJ. Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness. Am J Epidemiol 2016;184:345-53. [Crossref] [PubMed]
- Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013;31:2165-68. [Crossref] [PubMed]
- Westreich D, Hudgens MG. Invited Commentary: Beware the Test-Negative Design. Am J Epidemiol 2016;184:354-56. [Crossref] [PubMed]
Cite this article as: Jin P, Li J, Zhang X, Shen W, Duan K, Zhang L, Zhu F. Effectiveness of the inactivated enterovirus 71 vaccine in children aged 6–35 months: protocol for a multicentre, case-control phase IV clinical trial. J Public Health Emerg 2019;3:17.


