
Theoretical deposition of diesel exhaust particles in the respiratory tract of children
Introduction
Diesel exhaust particles (DEP) are commonly produced by combustion of diesel fuel and represent a complex mixture of variably sized and shaped aggregates (1-3). According to experimental studies DEP adopt aerodynamic diameters ranging from 100 to 250 nm (4-7), so that they may be attributed to the category of sub-micron particles. Particle size distribution of respective aggregates may be regarded as bimodal, whereby smaller particles formed by nucleation can be distinguished from those generated by agglomeration of the nuclei particles (8,9). As a special feature DEP are characterized by large surface areas facilitating the adsorption of diverse organic materials, among which several compounds have to be classified as mutagens and carcinogens (10,11).
Concerning the aerodynamic behaviour of DEP in the human respiratory tract, numerous experimental studies were conducted in the past decades. Whilst one category of experiments was mainly focused on the transport of sub-micron aggregates in artificial lung casts (12), another category grappled with the uptake of DEP into human probands by inhalation (13-15). During the past years experimental investigations dealing with DEP were accompanied by theoretical simulations describing the deposition of these particulate objects in the human respiratory tract. Such deposition models could continuously increase their predictive accuracy, because (I) more realistic models of lung architecture were used, (II) particle transport and deposition scenarios could be duplicated by more reliable mathematical equations and (III) complex particle shapes were subjected to more precise modeling procedures (16-25). Experimental and theoretical studies came to the uniform result that, under the assumption of normal (sitting) breathing conditions, DEP exhibit a preferential deposition in the upper and central airways. By increasing the inhalation flow rate, however, respective sub-micron aggregates undergo an enhanced transport to the distal lung region, finally resulting in their intensified deposition in the alveolar structures (20-27).
Experimental and epidemiological studies of DEP deposition in the human respiratory tract were mostly limited to adults hitherto, although children of different age may be also exposed to high extents to these particulate substances. Numerous theoretical investigations on particle behaviour in children’s lungs could furnish proof that particle deposition patterns of adult and subadult probands are certainly marked by remarkable similarities, but intensities of regional and local particle accumulations partly exhibit great discrepancies. In general, particle deposition produced by a single breath cycle is much lower in infants and children than in adults. These age-related differences of deposition, however, can be traced back to several reasons, among which age-specific lung morphometry and individual breathing habits may be regarded as most essential (28-35).
In the present contribution deposition of DEP in the lungs of children was subjected to a more detailed theoretical investigation. Thereby, two different age groups, i.e., 5-year-old and 10-year-old probands, were distinguished and related lung morphometries were generated by appropriate scaling procedures. Besides total (extrathoracic and thoracic) deposition of sub-micron aggregates, also regional (tubular, alveolar) and airway generation-specific deposition were modeled in order to obtain as much theoretical information on the intrapulmonary behaviour of this particle class as possible. Model simulations were conducted for two different breathing scenarios including sitting breathing on the one hand and light-exercise breathing on the other (36). The contents presented in this study may be regarded as important for several reasons:
- There are only few investigations dealing with the deposition of sub-micron particles in children’s lungs, so that any further increase of knowledge with regard to this topic can be evaluated as highly desirable.
- DEP and their effect as triggers of various lung diseases stand in the focus of extensive medical discussion. Here, theoretical deposition data for children may offer valuable support.
- A main question concerns the concrete change of particle deposition during lung development in early to intermediate phases of childhood. This problem can be partly solved with theoretical approaches.
Methods
Generation of randomly shaped DEP aggregates
The mathematical algorithm standing behind the construction of sub-micron aggregates was described in detail in previous publications (37-39), so that only most essential features will be outlined in this contribution. In general, DEP are thought to consist of a certain number of spherical components with equal size. In addition, they preferably form so-called isometric clusters, which are characterized by equal extension along the three axes of a Cartesian coordinate system. Generation of random aggregate shapes was conducted by means of the computer code AGGREGATE (39). The program is based on a random walk algorithm allowing the arbitrary arrangement of spherical components along the three coordinate axes. Thereby, for the addition of each component a specific construction routine determining the exact position of this element is passed through. The number of repetitions corresponds with the amount of spherical units included in the modeled aggregate.
For each aggregate constructed in the way described above essential aerodynamic parameters like dynamic shape factors and particle-related diameters were calculated by application of well-validated mathematical equations (40-42). Since DEP commonly cover a size range between 100 and 250 nm (see above), spherical units measuring 20 nm in diameter were used and randomly arranged to respective isometric clusters. The number of components included in the DEP aggregates varied between 100 (smallest size class) and 10,000 (largest size class). For all particles simulated with the help of the computer routine a physical density of 2.0 g cm−3 was assumed.
Deposition of computer-generated DEP aggregates in the human respiratory tract
For deposition modeling of DEP well-validated computer routines (15-25,37-39) were used, which are founded upon the following theoretical assumptions: (I) a stochastic structure of the tracheobronchial tree, (II) random selection of particle paths through the bronchial and bronchiolar airway sequences, (III) deposition of inhaled aggregates by four different mechanisms (Brownian motion, inertial impaction, interception, sedimentation) that are defined by empirical or analytical formulae (15-25). In order to obtain reliable statistics of particle transport and deposition in single lung structures, the Monte Carlo technique as well as the method of statistical weights allowing the calculation of multiple deposition events for a single particle were applied (43,44).
Results obtained from the modeling process included total, regional (i.e., tubular and alveolar) as well as local (i.e., airway generation-specific) deposition data of variably sized DEP aggregates (aerodynamic diameters: 100, 150, 200, 250 nm). Respective deposition scenarios were computed for two different breathing conditions, i.e., sitting breathing and light-exercise breathing, whereby in the second case tidal volume is increased by 60% and breath-cycle time is declined by 40% (28-36).
Theoretical approach to the lung morphometry of children with different age
Lung dimensions and related breathing parameters of 5-year-old and 10-year-old children were approximated by means of the logarithmic and exponential regression functions introduced in previous publications (45-50). In general, lung size of a 5-year-old child amounts to 51.7% of the respective size measured in an adult. In a child with an age of 10 years, size of the respiratory tract is increased to 66.5% of the adult dimensions, so that a significant morphometric difference between the two age categories used in this study can be recognized. According to the computed regression equations functional residual capacity (FRC) is enhanced from 767 cm3 (5 y) to 1,730 cm3 (10 y), whereas the tidal volume exhibits an increase from 267 to 461 cm3. Breathing frequency, on the other hand, is declined from 30 to 22.4 min−1, resulting in breath-cycle times of 2.0 and 2.5 s, respectively. For both age categories the complete absence of any breath-hold can be assumed.
Results
Total and regional deposition of DEP in children with different age
Under the assumption of sitting breathing conditions total deposition exhibits a significant decline with increasing DEP size in both age groups. Whilst for children with an age of 5 y deposition decreases from 41.5% (100 nm) to 24.1% (250 nm), in the respiratory tract of 10-year-old children deposition performs a respective decrease from 44.2% (100 nm) to 25.5% (250 nm). In general total deposition of DEP in the older age group surpasses that in the younger age group by 1.4% to 2.7% (Figure 1A).
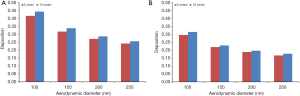
Under the assumption of light-exercise breathing those particle size-dependent deposition trends observed for sitting breathing are confirmed to a large extent. Within a group of 5-year-old children, total deposition declines from 29.5% (100 nm) to 16.6% (250 nm), whereas for 10-year-old children a decrease of deposition from 31.4% (100 nm) to 17.7% (250 nm) can be observed. Differences between the age groups amount to 1.1% to 1.9% and are therefore a little bit lower than for sitting breathing (Figure 1B). Enhancement of the breathing intensity due to increased physical activity commonly results in a remarkable decline of total deposition of DEP which ranges from 7% to 12%.
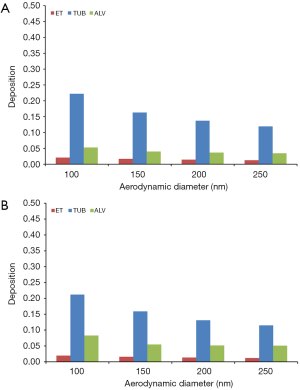
Concerning the deposition of DEP in different regions of the respiratory tract (extrathoracic, tubular, alveolar) similar trends as noted for total deposition can be recognized. For sitting breathing extrathoracic deposition becomes reduced from 11.1% (100 nm) to 7.04% (250 nm) in 5-year-old children and from 10.0% (100 nm) to 6.44% (250 nm) in 10-year-old children. Tubular deposition decreases from 24.8% to 13.6% in younger probands and from 25.4% to 14.3% in older ones, whereas alveolar DEP accumulation is subject to a reduction from 5.63% to 3.46% in children with an age of 5 y and from 8.71% to 4.70% in children with an age of 10 y (Figure 2A,B).
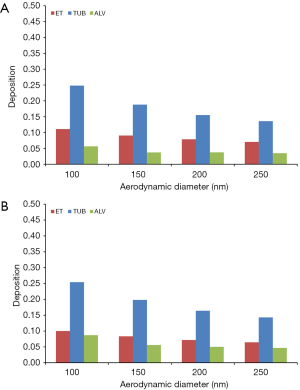
In the case of young probands performing light physical exercises, extrathoracic deposition exhibits a decrease from 20.9% to 12.7% (5 y) and from 19.3% to 11.8% (10 y). Tubular deposition is characterized by a reduction from 22.2% to 11.9% (5 y) and from 21.2% to 11.5% (10 y), whilst alveolar deposition develops from 5.26% to 3.46% (5 y) and from 8.29% to 5.09% (10 y) (Figure 3A,B).
Deposition of DEP in single airway generations
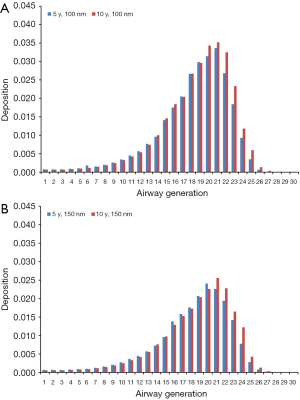
Modeling results obtained for the case of sitting breathing are summarized in Figures 4 and 5. For DEP with an aerodynamic diameter of 100 nm generation-specific deposition varies from 0.0032% (generation 27) to 3.55% (generation 20) in the younger age group and from 0.011% (generation 27) to 3.90% (generation 20) in the older age group (Figure 4A). Diesel aggregates with an aerodynamic diameter of 150 nm deposit with 4.07·10−4% (generation 26) to 2.66% (generation 19) in 5-year-old children and with 0.0014% (generation 28) to 3.02% (generation 20) in 10-year-old children (Figure 4B). In the case of DEP measuring 200 nm in size, local deposition ranges from 4.26·10−5% (generation 27) to 2.27% (generation 20) in younger probands and from 6.07·10−4% (generation 27) to 2.50% (generation 21) in older ones (Figure 5A). Aggregates with an aerodynamic diameter of 250 nm exhibit a local deposition varying between 5.51·10−4% (generation 27) and 1.94% (generation 19) in 5-year-old children as well as from 9.53·10−4% (generation 28) to 2.22% in 10-year-old children (Figure 5B).
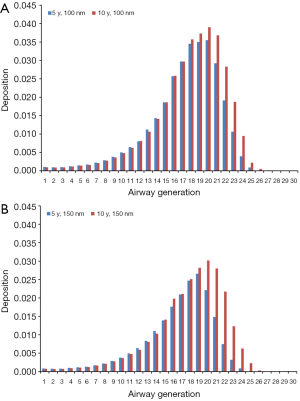
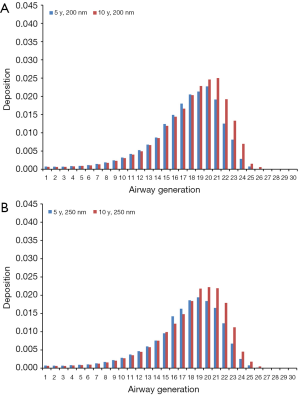
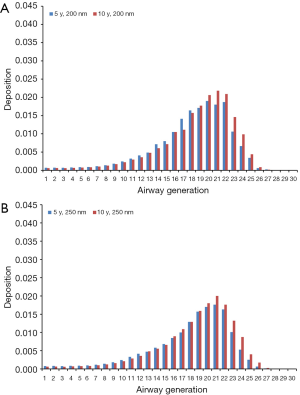
Theoretical results obtained for the case of light-exercise breathing are summarized in Figures 6 and 7. Diesel particles adopting an aerodynamic diameter of 100 nm deposit with 2.49·10−4% (generation 28) to 3.36% (generation 21) in 5-year-old children and with 0.0023% (generation 28) to 3.52% (generation 21) in 10-year-old children (Figure 6A). Regarding DEP with an aerodynamic diameter of 150 nm, local deposition varies between 2.33·10−4% (generation 28) and 2.41% (generation 20) in younger probands and between 6.12·10−5% (generation 28) and 2.56% (generation 21) in older ones (Figure 6B). For DEP with an aerodynamic diameter of 200 nm generation-related deposition is subject to a variation ranging from 3.42·10−5% (generation 28) to 1.90% (generation 20) in children with an age of 5 y and from 0.00112% (generation 28) to 2.18% (generation 21) in probands with an age of 10 y (Figure 7A). With regard to DEP reaching a size of 250 nm deposition values between 1.15·10−10% (generation 28) and 1.76% (generation 21) can be observed in younger probands, whereas in older ones respective values range from 4.51·10−4% (generation 28) to 2.0% (generation 21; Figure 7B).
Discussion
The theoretical results presented in this contribution underline the hypothesis, according to which DEP are deposited in children’s lungs in considerable amounts (28-36). Concerning total deposition of aggregates ranging in size from 100 to 250 nm several interesting observations could be made: (I) independent of the age group, deposition values exhibit a negative correlation with the aerodynamic diameter of the particulate constructs. This means that larger DEP produce higher exhalation fractions than smaller ones. (II) Direct comparison of the two age groups shows slightly higher deposition rates per breath-cycle in 10-year-old children than in 5-year-old probands. (III) By increasing the breathing intensity (switch from sitting to light-exercise inhalation) deposition of DEP per breath-cycle is subject to a significant decrease. With regard to point (I) it has to be mentioned that particles <100 nm are mainly affected by diffusion processes, finally resulting in their collision with airway and alveolar walls. Particles ranging in size from 100 to 500 nm are characterized by minimal effects of single deposition mechanisms, so that their chance of being exhaled is subject to a continuous growth (20-27,45-50). The result summarized under point (II) can be led back to the circumstance that tidal volume of older children exceeds that of younger ones by about 73%, resulting in a larger uptake of DEP by inhalation. Due to the much greater lung size occurring in 10-year-old probands only a small percentage of these surplus particles is also involved into respective deposition scenarios (28-36). Enhancement of the breathing intensity noted under point (III) correlates with a partly dramatic increase of the axial transport velocity of inhaled particles, especially in the upper airways of the respiratory tract. This phenomenon, however, diminishes the probability of DEP deposition by lateral diffusion processes noted above (45-50). It has to be stated emphatically that all computations are related to single breath-cycles. If deposition rates of DEP are calculated for a certain period of time (e.g., 24 h) due to long-term exposure, younger children are exposed to much higher hazards than older children due to the differences in breathing frequency. Also any increase of physical activity, which may be advantageous with regard to a single breath, finally produces higher deposition rates.
Regional deposition of DEP is generally characterized by a predominance of particle accumulation in the tubular structures of the respiratory tract, whereas the walls of the extrathoracic airways and alveoli are hit by much lower particle fractions. Similar to total deposition also regional deposition fractions are declined with growing particle size but slightly increased from younger to older children. Enhancement of physical activity results in a decrease of DEP deposition in all lung regions, whereby respective accumulation of particles is most significantly declined in the extrathoracic region. In the tubular and alveolar lung structures activity-related reduction of particle deposition turns out more moderate (28-36). According to the theoretical computations of this study the extrathoracic and thoracic airways represent the main deposition targets of DEP. This phenomenon may be largely led back to the circumstance that diffusion processes exerting on the inhaled particulate mass exhibit highest efficiency in small tubular structures (35-42). As an essential consequence of the calculation results particle doses per unit area are much higher in bronchial and bronchiolar airways than they are in the alveolar spheres. Hence, the risk for DEP occurring as triggers of diseases in the airway system is increased, whilst the probability of alveolar insufficiencies induced by DEP seems to be rather limited.
According to the theoretical simulations, highest deposition probabilities of DEP can be predicted for more peripheral airway generations (16 to 22). Local deposition of these sub-micron particles is again influenced by the aerodynamic diameter of the aggregates, lung morphometry and physical activity. Whilst larger particles commonly exhibit lower deposition rates than smaller ones, lung size and mode of breathing have two consequences for deposition: (I) in analogy to total and regional deposition, local particle accumulation is negatively correlated with both parameters. (II) The mode of deposition is shifted towards more distal airway generations. The phenomenon stated under point (II) has to be repeatedly understood as a result of higher lateral diffusion distances occurring in larger lungs (10-year-old children) with respect to smaller ones (5-year-old children) (28-36).
Conclusions
The results presented in this study lead to several important conclusions:
- DEP are deposited in children’s lungs in sufficient amount, so that they have to be classified as serious environmental health hazards.
- Although older children take up more DEP per breath-cycle, over longer periods of exposure younger children may accumulate much more particles in their lungs and thus represent the more endangered age group.
- Deposition intensity of DEP commonly declines with increasing size of the particulate aggregates. Thus, agglomerates seem to be less dangerous than particles emerging from nucleation.
- Enhanced physical activity reduces the deposition intensity of DEP per breath cycle, but increases the amount of accumulated particulate mass over a longer time period.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2019.09.01). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Groblicki PJ. Particle Size Variation in Diesel Car Exhaust. SAE Technical Paper Series No. 790421. Warrendale: Society of Automotive Engineers, 1979.
- Dolan DF, Kittelson DB, Pui DYH. Diesel Exhaust Particle Size Distribution Measurement Techniques. SAE Technical Paper Series No. 870254. Warrendale: Society of Automotive Engineers, 1980.
- Obert EF. Internal Combustion Engines and Air Pollution, 3rd ed. New York: Harper and Row, 1973.
- Ullman TL. Investigation of the Effects of Fuel Composition on Heavy Duty Diesel Engine Emissions. SAE Technical Paper No. 892072. Society of Automotive Engineers, 1989.
- McClellan RO. Toxicological effects of emissions from diesel engines. Dev Toxicol Environ Sci 1986;13:3-8. [PubMed]
- Williams RL. Diesel particulate emissions: Composition, concentration, and control. Dev Toxicol Environ Sci 1982;10:15-32. [PubMed]
- Baumgard KJ, Johnson JH. The effect of Fuel and Engine Design on Diesel Exhaust Particle Size Distributions. SAE Technical Paper Series No. 960131. Warrendale: Society of Automotive Engineers, 1996.
- IARC. Diesel and gasoline engine exhausts. In: Diesel and Gasoline Engine Exhausts and Some Nitroarenes. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, vol. 46. Lyon: International Agency for Research on Cancer, 1989.
- NTP. Report on Carcinogens Background Document for Diesel Exhaust Particulates. Research Triangle Park: National Toxicology Program, 2000.
- Garshick E, Laden F, Hart JE, et al. Lung cancer and vehicle exhaust in trucking industry workers. Environ Health Perspect 2008;116:1327-32. [Crossref] [PubMed]
- Neumeyer-Gromen A, Razum O, Kersten N, et al. Diesel motor emissions and lung cancer mortality—results of the second follow-up of a cohort study in potash miners. Int J Cancer 2009;124:1900-6. [Crossref] [PubMed]
- Penconek A, Arkadiusz M. Deposition of diesel exhaust particles from various fuels in a cast of human respiratory system under two breathing patterns. J Aerosol Sci 2013;63:48-59. [Crossref]
- Morawska L, Hofmann W, Hitchins-Loveday J, et al. Experimental study of the deposition of combustion aerosols in the human respiratory tract. J Aerosol Sci 2005;36:939-57. [Crossref]
- Löndahl J, Massling A, Swietlicki E, et al. Experimentally determined human respiratory tract deposition of airborne particles at a busy street. Environ Sci Technol 2009;43:4659-64. [Crossref] [PubMed]
- Rissler J, Swietlicki E, Bengtsson A, et al. Experimental determination of deposition of diesel exhaust particles in the human respiratory tract. J Aerosol Sci 2012;48:18-33. [Crossref]
- Sturm R. Theoretical deposition of random walk-generated nanoaggregates in the lungs of healthy males and females. J Publ Health Emerg 2018;2:4. [Crossref]
- Sturm R. Theoretical deposition of variably sized platelets in the respiratory tract of healthy adults. AME Med J 2018;3:61. [Crossref]
- Sturm R. Deposition of diesel exhaust particles in the human lungs: theoretical simulations and experimental data. J Publ Health and Emerg 2017;1:7.
- Sturm R, Hofmann W. A theoretical approach to the deposition and clearance of fibers with variable size in the human respiratory tract. J Hazard Mater 2009;170:210-8. [Crossref] [PubMed]
- Sturm R. Nanotubes in the respiratory tract - Deposition modeling. Z med Phys 2015;25:135-45. [Crossref] [PubMed]
- Sturm R. Inhaled nanoparticles. Phys Today 2016;69:70-1. [Crossref]
- Sturm R. A stochastic model of carbon nanotube deposition in the airways and alveoli of the human respiratory tract. Inhal Toxicol 2016;28:49-60. [Crossref] [PubMed]
- Sturm R. Theoretical deposition of carcinogenic particle aggregates in the upper respiratory tract. Ann Transl Med 2013;1:25. [PubMed]
- Sturm R. Spatial visualization of theoretical nanoparticle deposition in the human respiratory tract. Ann Transl Med 2015;3:326. [PubMed]
- Sturm R. Deposition of ultrafine particles with various shapes in the human alveoli – a model approach. Comp Math Biol 2016;5:4.
- Sturm R. A theoretical approach to the deposition of cancer-inducing asbestos fibers in the human respiratory tract. TOLCJ 2009;2:1-11. [Crossref]
- Sturm R, Hofmann W. Modellrechnungen zur Deposition nicht-sphärischer Teilchen in den oberen Luftwegen der menschlichen Lunge. Z Med Phys 2009;19:38-46. [Crossref] [PubMed]
- Sturm R. Theoretical models of carcinogenic particle deposition and clearance in children’s lungs. J Thorac Dis 2012;4:368-76. [PubMed]
- Sturm R. Theoretical deposition of nanotubes in the respiratory tract of children and adults. Ann Transl Med 2014;2:6. [PubMed]
- Sturm R. Bioaerosols in the lungs of subjects with different ages-part 1: deposition modeling. Ann Transl Med 2016;4:211. [Crossref] [PubMed]
- Sturm R. Bioaerosols in the lungs of subjects with different ages-Part 2: clearance modeling. Ann Transl Med 2017;5:95. [Crossref] [PubMed]
- Sturm R. Age-dependence and intersubject variability of tracheobronchial particle clearance. Pneumon 2011;4:77-84.
- Sturm R, Hofmann W 3D. -Visualization of particle deposition patterns in the human lung generated by Monte Carlo modeling: methodology and applications. Comput Biol Med 2005;35:41-56. [Crossref] [PubMed]
- Sturm R, Hofmann W. Stochastisches Modell zur räumlichen Visualisierung von Teilchendepositionsmustern in der Lunge und ihre Bedeutung in der Lungenmedizin. Z Med Phys 2006;16:140-7. [Crossref] [PubMed]
- Sturm R. A computer model for the simulation of nonspherical particle dynamics in the human respiratory tract. Phys Res Int 2012;1:11.
- International Commission on Radiological Protection (ICRP). Human respiratory tract model for radiological protection, Publication 66. Oxford: Pergamon Press, 1994.
- Sturm R. Theoretical models for dynamic shape factors and lung deposition of small particle aggregates originating from combustion processes. Z med Phys 2010;20:226-34. [Crossref] [PubMed]
- Sturm R. Lung deposition of particle aggregates generated with a random walk model. Comp Math Biol 2017;6:1.
- Sturm R. Computer-aided generation and lung deposition modeling of nano-scale particle aggregates. Inhal Toxicol 2017;29:160-8. [Crossref] [PubMed]
- Fuchs NA. The Mechanics of Aerosols. New York: Pergamon Press, 1964.
- Kasper G. Dynamics and measurement of smokes. I Size characterization of nonspherical particles. Aerosol Sci Technol 1982;1:187-99. [Crossref]
- Willeke K, Baron PA. Aerosol measurement. New York, NY: John Wiley, 1993.
- Koblinger L, Hofmann W. Monte Carlo modeling of aerosol deposition in human lungs. Part I: Simulation of particle transport in a stochastic lung structure. J Aerosol Sci 1990;21:661-74. [Crossref]
- Hofmann W, Sturm R, Winkler-Heil R, et al. Stochastic model of ultrafine particle deposition and clearance in the human respiratory tract. Radiat Prot Dosimetry 2003;105:77-80. [Crossref] [PubMed]
- Sturm R. Theoretical and experimental approaches to the deposition and clearance of ultrafine carcinogens in the human respiratory tract. Thorac Cancer 2011;2:61-8. [Crossref] [PubMed]
- Sturm R. A computer model for the simulation of fiber-cell interaction in the alveolar region of the respiratory tract. Comput Biol Med 2011;41:565-73. [Crossref] [PubMed]
- Sturm R, Hofmann W. A computer program for the simulation of fiber deposition in the human respiratory tract. Comput Biol Med 2006;36:1252-67. [Crossref] [PubMed]
- Sturm R. Theoretical approach to the hit probability of lung-cancer-sensitive epithelial cells by mineral fibers with various aspect ratios. Thoracic Cancer 2010;1:116-25. [Crossref] [PubMed]
- Sturm R. Modeling the deposition of bioaerosols with variable size and shape in the human respiratory tract–A review. J Adv Res 2012;3:295-304. [Crossref]
- Sturm R. Inhalation of nanoplatelets - theoretical deposition simulations. Z Med Phys 2017;27:274-84. [Crossref] [PubMed]
Cite this article as: Sturm R. Theoretical deposition of diesel exhaust particles in the respiratory tract of children. J Public Health Emerg 2019;3:12.


