
Comparison of plasma lipids changes after middle-distance running in euglycemic and diabetic subjects
Introduction
Several lines of evidence now attest that regular performance of aerobic physical exercise is pivotal for preserving or improving health and fitness, and shall hence be part of public health policies. This conclusion is actually supported by a kaleidoscope of metabolic changes that physical activity induces, and which translate into a consistently lower risk of developing cardiovascular, metabolic, musculoskeletal and even malignant disorders (1). Although the vast majority of favorable cardiovascular changes catalyzed by endurance running emerge as long-term (chronic) effects on hemostasis (2) and on the lipid profile (3,4), the acute impact of endurance exercise on plasma lipids remains controversial in the current scientific literature. Many previous endurance trials have frequently reported inconsistent variations of pro-atherogenic [i.e., low-density lipoprotein-cholesterol (LDL-C) and triglycerides] or anti-atherogenic [high-density lipoprotein-cholesterol (HDL-C)] lipoprotein fractions, with ample inter-study variations (5-7). It is then noteworthy that most currently available studied were based on long-distance running (i.e., marathon or ultra-marathon), whilst data on acute effect of medium-distant running (i.e., between 10–21 km), which is a more common recreational distance typically run by a larger number of recreational athletes, are completely lacking to the best of our knowledge. This seems a notable drawback, since lipid metabolism is directly dependent on running distance, with most changes becoming evident when covering running distances comprised between 10–21 km (8). Even less defined is the acute impact of endurance running on the lipid profile of diabetic patients, since information on this condition is completely lacking compared to euglycemic subjects. Therefore, this study was aimed to investigate the acute changes of plasma lipids after middle-distance running, and compare these variations between euglycemic and diabetic subjects.
Methods
Study design
The present research was part of an event called “Run For Science”, which is annually held under the auspices of University of Verona (Verona, Italy). Eleven male euglycemic amateur runners (mean age 41±6 years) and 9 male diabetic amateur runners (4 with type 1 and 5 with type 2 diabetes; mean age 55±14 years) voluntarily engaged in a 21.1-km (i.e., half-marathon) running trial. The run started at around 7:30 AM on a mostly flat circuit (maximal slope, 1.8%), as thoughtfully described elsewhere (9). All subjects belonged to an amateur running team, and were regularly engaged in recreational running (at least 2 times a week). All subjects abstained from eating solid foods and strenuous physical activity for not less than 8 and 48 hours before the start of the trial, respectively. All diabetics were asked to continue their regular therapy with insulin and/or oral diabetes type 2 drugs throughout the period before the run. Athletes were only allowed to drink water (at libitum) throughout the running trial. Blood was drowned into evacuated blood collection tubes containing lithium-heparin as additive (Vacutest Kima s.r.l., Padova, Italy), before the start of the run and immediately after all subjects completed the trial. The blood samples were then conveyed to the service of Laboratory Medicine of the University Hospital of Verona (Verona, Italy).
Analysis of plasma lipids
All blood samples were immediately centrifuged at 1,300×g for 15 min at room temperature immediately after arrival in the laboratory. Lithium-heparin plasma was separated from the blood cell layer underneath and immediately analyzed. All measurements were performed in a single batch, with duplicate measures (final results were expressed as mean of the two measurements). Total cholesterol was measured with an enzymatic colorimetric assay (cholesterol oxidase reaction), HDL-C was assayed with a homogeneous enzymatic colorimetric assay (preliminary reaction with polyanions and detergent, followed by cholesterol oxidase reaction), whilst triglycerides were measured with an enzymatic colorimetric assay (glycerophosphate oxidase reaction). The plasma concentration of LDL-C was finally calculated according to the validated formula of Friedewald et al. (10). Post-run values were always corrected for the plasma volume change (PVC), according to the formula of Dill and Costill (11). All measurements were performed using the same Roche Cobas 6000 analyzer (Roche Diagnostics, Basel, Switzerland), during an identical analytical session. The within-run imprecision of all these assays is <1%, as quoted by the manufacturer.
Statistical analysis
Final results were normally distributed (according to Kolmogorov-Smirnov test), and were hence reported as mean and standard deviation (SD). The significance of differences was evaluated with paired or unpaired Student’s t-test, when appropriate. The level of statistical significance was set at P<0.05, and the statistical analysis was performed with Analyse-it (Analyse-it Software Ltd., Leeds, UK). All athletes signed a written informed consent for participating to this trial. The study was approved by the local ethical committee (Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy; protocol number 165038) and performed in accordance with the Helsinki Declaration of 1975.
Results
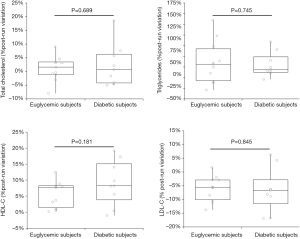
Although diabetic subjects were on average 14 years older than euglycemic runners, the baseline values of all plasma lipids were found to be non-significantly different between diabetic and euglycemic subjects, as shown in Table 1. All athletes successfully completed the 21.1-km running trial, with running pace comprised between 9.6–12.8 km/h. The running pace did not significantly differ between diabetics and euglycemic subjects (P=0.131). The acute effect of the run on plasma volume and blood lipids is shown in Table 2. The plasma volume significantly increased by approximately 6.9% (P=0.032) in euglycemic subjects, whilst it remained virtually unchanged in diabetics (−2.1%; P=0.466). In both categories of subjects the values of LDL-C was found to be significantly decreased after the run, while those of HDL-C and triglycerides were significantly increased (Table 2). Total cholesterol remained unchanged after the run in both categories of subjects. Notably, no significant difference was noted between diabetic and euglycemic subjects in the percent post-run variation of all lipoprotein fractions after the running trial (Figure 1).
Table 1
| Parameter | Euglycemic subjects | Diabetic subjects | P |
|---|---|---|---|
| Number | 11 | 9 | |
| Age (years) | 41±6 | 55±14 | 0.012 |
| Total cholesterol (mmol/L) | 4.82±0.82 | 4.19±0.60 | 0.072 |
| LDL-C (mmol/L) | 2.78±0.75 | 2.31±0.52 | 0.135 |
| HDL-C (mmol/L) | 1.65±0.24 | 1.47±0.44 | 0.257 |
| Triglycerides (mmol/L) | 0.85±0.30 | 1.04±0.50 | 0.311 |
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Table 2
| Parameter | Euglycemic subjects | Diabetic subjects | |||
|---|---|---|---|---|---|
| Value | P vs. baseline | Value | P vs. baseline | ||
| Plasma volume | 6.9±8.4% | 0.032 | −2.1±6.6% | 0.466 | |
| Total cholesterol | 1±4% | 0.335 | 2±8% | 0.455 | |
| LDL-C | −6±5% | 0.001 | −6±7% | 0.039 | |
| HDL-C | 6±4% | 0.001 | 9±7% | 0.004 | |
| Triglycerides | 36±49% | 0.032 | 30±28% | 0.010 | |
All lipid values have been corrected for the plasma volume change. LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Discussion
Although it is now undeniable that performing regular physical exercise is associated with many positive changes of lipid profile on the long-term (12), there is less convincing information on acute changes of plasma lipids after endurance running and, especially, no data has been published on the effect of middle-distance running (i.e., between 10–21 km) on the lipid profile in both euglycemic and diabetic subjects.
Some innovative results have emerged from this study, which may have some interesting and practical clinical implications. We first showed that middle-distance running induces some acute favorable changes in the lipoprotein profile. Namely, the pro-atherogenic LDL-C fraction was found to be significantly decreased by approximately 6%, whilst the anti-atherogenic HDL-C fraction displayed an opposite trend, with a 6–9% acute increase of its values (Table 2). Overall, this can be classified as a beneficial shift of lipid profile towards a more favorable condition, thus providing additional evidence to that already published in marathon and ultra-marathon runners. Kuusi et al studied 20 healthy amateur runners who participated to the First North Karelian Heart Marathon, and showed that the HDL-C fraction significantly increased by approximately 7% at the end of the run (5). Unlike our findings, however, they failed to find any significant variation of the LDL-C fraction, which remained virtually unchanged after the end of the marathon. Notably, no significant variations were also observed for total cholesterol and triglycerides. Similar results were reported by Liu et al. (6), who also studied 11 well-trained male runners participating to the Helsinki City Marathon, and failed to find any significant variation in the lipid profile after completing the running distance except for HDL-C fraction, which increased by approximately 3%. In an ensuing study, Emed et al. studied 16 male athletes who took part to an ultramarathon run (mean distance covered, 134 km) (7), and found that neither total cholesterol or LDL-C, HDL-C and triglyceride values significantly changed after this strenuous trial, thus concluding that the lipid profile was unaltered by strenuous, long-distance running.
A second remarkable aspect emerged from our study. The acute beneficial changes of physical exercise on the lipid profile were found to be virtually identical between euglycemic and diabetic subjects, thus providing further support and reinforcing the recommendations of fostering physical activity programs in prevention and management of diabetes (13). Interestingly, our data complement those already published on the beneficial influence of a single exercise session in diabetics for increasing bioavailability of nitric oxide, eliciting reduction of blood pressure, enhancing carbohydrate oxidation and fat oxidation, thus ultimately ameliorating insulin sensitivity and glucose tolerance (14). All these acute changes, combined with evidence that middle-distance running would actually contribute to acutely ameliorate the lipid profile, persuade us to suggest that this form of exercise may be highly indicated in diabetics.
Conclusions
In conclusion, the results of our original study convincingly show for the first time that middle-distance running elicits favorable acute changes of the lipid profile, both in euglycemic and diabetic subjects. Therefore, this form of common and sustainable endurance exercise shall be further fostered for purposes of public health promotion and improvement.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2019.06.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the local ethical committee (Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy; protocol number 165038) and performed in accordance with the Helsinki Declaration(as revised in 1975). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Schena F, Guidi GC. Health benefits of physical activity. CMAJ 2006;175:776-author reply 777. [Crossref] [PubMed]
- Lippi G, Maffulli N. Biological influence of physical exercise on hemostasis. Semin Thromb Hemost 2009;35:269-76. [Crossref] [PubMed]
- Lippi G, Schena F, Salvagno GL, et al. Comparison of the lipid profile and lipoprotein(a) between sedentary and highly trained subjects. Clin Chem Lab Med 2006;44:322-6. [Crossref] [PubMed]
- Kelley GA, Kelley KS. Aerobic exercise and lipids and lipoproteins in men: a meta-analysis of randomized controlled trials. J Mens Health Gend 2006;3:61-70. [Crossref] [PubMed]
- Kuusi T, Kostiainen E, Vartiainen E, et al. Acute effects of marathon running on levels of serum lipoproteins and androgenic hormones in healthy males. Metabolism 1984;33:527-31. [Crossref] [PubMed]
- Liu ML, Bergholm R, Mäkimattila S, et al. A marathon run increases the susceptibility of LDL to oxidation in vitro and modifies plasma antioxidants. Am J Physiol 1999;276:E1083-91. [PubMed]
- Emed LG, Passaglia DG, Guerios ST, et al. Acute modification in plasma lipid levels in ultramarathon runners. J Sports Sci 2016;34:1657-61. [Crossref] [PubMed]
- Kokkinos PF, Holland JC, Narayan P, et al. Miles run per week and high-density lipoprotein cholesterol levels in healthy, middle-aged men. A dose-response relationship. Arch Intern Med 1995;155:415-20. [Crossref] [PubMed]
- Lippi G, Schena F. Run for Science (R4S): the history of a successful project of precision and laboratory medicine in sport and exercise. J Lab Precis Med 2017;2:11. [Crossref]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502. [PubMed]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 1974;37:247-8. [Crossref] [PubMed]
- Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med 2014;44:211-21. [Crossref] [PubMed]
- Thent ZC, Das S, Henry LJ. Role of exercise in the management of diabetes mellitus: the global scenario. PLoS One 2013;8:e80436 [Crossref] [PubMed]
- Asano RY, Sales MM, Browne RA, et al. Acute effects of physical exercise in type 2 diabetes: A review. World J Diabetes 2014;5:659-65. [Crossref] [PubMed]
Cite this article as: Lippi G, Tarperi C, Salvagno GL, Benati M, Danese E, Montagnana M, Moghetti P, Schena F. Comparison of plasma lipids changes after middle-distance running in euglycemic and diabetic subjects. J Public Health Emerg 2019;3:10.


