
Writing a better research article
Introduction
“Write and rewrite, rewrite again, and then revise!”—Morris Fishbein, MD [1889–1976], Editor, Journal of the American Medical Association, 1924 to 1950 (1).
At the core of all sciences are four questions: Why did you start? What did you do? What did you find? What does it mean (2)? These four questions also provide the format for most articles reporting scientific research because they are answered in the introduction, the methods, the results, and the discussion, respectively. Since the 1970s, this format, known by its initials as the “IMRAD” format, has been an international standard for reporting research in scientific journals (3).
All journal editors will tell you that they want to publish research that is new, high-quality, important, and clearly reported (4). They will also tell you that they want to receive manuscripts prepared according to their journal’s instructions for authors. This advice is repeated in many of the articles in this Focused Issue of the Journal because it is so important. Other things being equal, manuscripts that conform to the instructions for authors are more likely to be considered seriously because they save the journal time, effort, and money. (See “Choosing and Communicating with Journals” elsewhere in this issue of the Journal).
In this article, I describe several techniques that will help you prepare each of the IMRAD sections, as well as the other standard parts of a scientific article. Other articles in this issue of the Journal address many of these topics in more detail. Several books also provide excellent guidance in preparing and publishing scientific articles (5-10).
Improving the title
The title is the most important part of the article because it connects your research with readers who might be interested in learning about it.
Scientific articles usually have one of three types of titles: declarative, interrogative, and informative. A declarative or headline title is a complete sentence that states the results:
- Lactoferrin efficiently counteracts the inflammation-induced changes of the iron homeostasis system in macrophages.
- Vitamin D deficiency is common and associated with overweight in Mexican children.
Declarative titles are often found in articles reporting basic research (the first example above) but are discouraged in articles reporting clinical research (the second example above). Basic science articles are read almost exclusively by basic scientists, who know that a result mentioned in the title is not necessarily true under all conditions and that it was affected by the strengths and limitations of the research methods. Public health and clinical journals, however, are often read by more diverse audiences: physicians, nurses, allied health personnel, patients and their families, newspaper reporters, lawyers, and so on. The concern is that readers unfamiliar with clinical research may believe that the result stated in a declarative title is an established fact, rather than one piece of evidence in understanding a complex process. For this reason, declarative titles are far less common in clinical and public health journals (11).
A second type of title is the “interrogative title”, which is phrased as a question:
- Is mannitol the treatment of choice for patients with ciguatera fish poisoning?
- Is tea tree oil effective at eradicating methicillin-resistant staphylococcus aureus colonization?
Interrogative titles do not provide a lot of information—including the point of the article—they just call attention to the issue addressed in an article. As a result, they are not usually suitable for articles reporting research and are best limited to editorials.
The most common type of title in articles reporting public health and epidemiological research is the “informative title”, which typically identifies the population, exposure, event, treatment, outcome, or relationship that was studied:
- Serum lead concentrations in Jamaican children with and without autism spectrum disorder.
- Predicting falls in institutionalized elderly people from the nursing diagnosis.
- Food insecurity as a predictor of domestic violence in Kazakhstan.
Consider including any or all of the following seven elements when writing a title: (I) the study setting, location, or both; (II) the patients, organism, event, or relationship studied; (III) the intervention, treatment, or exposure; (IV) the comparator or control group(s); (V) the outcomes or end points; (VI) the study design, and sometimes (VII) the time period or duration of the study (5). For example, suppose a study has the following characteristics:
- the setting and location: refugee settlements in Jordan;
- the patients or subjects: residents using public toilets;
- the intervention: self-disinfecting “smart toilets” using ultraviolet irradiation to kill bacteria;
- the control condition: regular manual toilet cleaning;
- the end point: Escherichia coli infections;
- the study design: randomized trial;
- the time period (probably not a factor in this study).
Now create a title, starting with all the elements, and then edit for clarity and length. Many journals limit titles to about 80 characters and spaces. If so, remove the least important elements until you have met the character limit.
- Effectiveness of “Smart Toilets” Using Ultraviolet Germicidal Irradiation vs Regular Cleaning for Reducing Escherichia coli Infections in Refugee Settlements in Jordan: A Randomized Trial [187 characters and spaces]
- Effectiveness of “Smart Toilets” Using Ultraviolet Irradiation for Reducing Escherichia coli Infections in Refugee Settlements [127 characters and spaces]
- Effectiveness of Self-Disinfecting Toilets for Reducing Escherichia coli Infections in Refugee Settlements [107 characters and spaces]
- Effectiveness of Self-Disinfecting Toilets for Reducing Escherichia coli Infections [84 characters and spaces]
The title is the part of the article most often read and often the only part read. For these reasons, you should take the time to write a good title.
Improving the abstract
The only purpose of an abstract is to help readers decide whether to read the full article (5). The three most common types of publication abstracts are descriptive, informative, and structured (meeting abstracts have different characteristics). Descriptive abstracts do not give results as much as they tell readers what topics are discussed in the full article. As a result, they are more often used for review articles rather than for articles reporting original research (the abstract for this article is descriptive, for example).
Most research articles use informative abstracts that have the four IMRAD parts: an introduction, methods, results, and conclusions (but not a full discussion). Informative abstracts give a brief background of the research, the major points of the methods, summary data for the primary end points, and the main conclusions. Many journals require structured abstract
The abstract is the second most often read part of the article (after the title), but it should be the last part written. Inaccuracies and inconsistencies between the abstract and the full article are very common—most often read of studies have found important discrepancies in between 30% to 60% of articles (13-16). Among the most common and serious errors is that the information in the abstract—sample sizes and characteristics, data, and even conclusions—differs from that in the article. Thus, using the same wording for the conclusions in both the abstract and the text is a good idea. Writing the abstract after finishing the article should improve consistency, but be sure to compare the two parts closely before submitting the article.
Improving the introduction: why did you start?
The introduction should prepare readers to understand why and how you did your research and tell them what to expect if they read your article. That is, a good introduction, especially after a good title and abstract, should let readers decide whether or not to read your article.
I recommend writing a four-part introduction (17).
In part 1, the background statement, orient readers to the area of public health of interest and give them the background they need to understand the problem.
In part 2, the problem statement, describe the nature and importance of the problem you studied. Identify the effects or implications of the problem, its scope and severity, and the populations affected. Convince readers that your research addresses an important issue. This information is often missing in scientific articles because authors assume—incorrectly—that readers will know what the research sought to do and why it was done.
In part 3, the activity statement, tell what you did to address the problem. Describe your hypothesis, how you studied the problem, and why you approached the problem the way you did. Convince readers that your research will adequately answer the questions associated with the problem.
In part 4, the forecasting statement, tell readers what to expect if they continue to read your article. By the end of the introduction, readers should be able to determine whether your article is likely to interest them.
The example below illustrates these four parts:
- [Part 1: Background statement]. Some ingredients in personal care products, such as skin care products, deodorants, and toothpaste, have been identified as “endocrine disruptors,” or molecules that can affect and interfere with the human hormonal system.
- [Part 2: Problem statement]. Many endocrine disruptors are easily absorbed into the subcutaneous tissue and blood, raising concerns about whether the use of personal care products can affect human health.
- [Part 3: Activity statement]. To determine the collective effect of these chemicals on human health, population studies must determine the association between the use of personal care products and hormonally related diseases. As a first step in this process, it is important to characterize heavy users of these products.
- [Part 4: Forecasting statement]. Here, we report our study of more than 100,000 women in the Norwegian Women and Cancer study in which we compared the lifestyle characteristics of heavy users of skin care products with those of non-users. We also assessed the association between the use of these products and the endocrine events in a woman’s life—menarche, pregnancy, breastfeeding, and menopause—which are important factors in hormonally related diseases.
One of the most common problems in writing introductions is that parts 1, 2, and 4 are incomplete or missing. Using the above introduction, an incomplete introduction might read as follows:
- [Incomplete introduction] Some ingredients in skin care products, deodorants, and toothpaste, may have molecules that can affect the human hormonal system. We studied heavy users of these products to see if their use was associated with hormonally related conditions.
Missing from this incomplete introduction is the key term, “endocrine disruptors”, and the facts that these compounds are found in many kinds of personal care products, not just in those listed; that they are absorbed through the skin and have raised health concerns; that the research is the first step in a larger research effort to identify specific health problems; that this research compared heavy users to nonusers of these products among 100,000 women in the Norwegian Women and Cancer study; and that the hormonally related conditions were menarche, pregnancy, breastfeeding, and menopause.
A good introduction should be long enough to do what it is supposed to do, as described above. Many introductions are too short because authors do not appreciate how important a good introduction can be. Length can also be affected by the conventions in a particular field of science: journals in the social sciences (including some nursing and public health journals) often include the literature review in the introduction, whereas those in the clinical sciences usually include the literature review in the discussion, for example.
Improving the methods: what did you do?
The purpose of the methods section is to tell readers how you went about answering your research question. Although many authors have been taught that the methods section should allow others to repeat their research, the reality is that the typical 3,000-word scientific article is usually not long enough to include this amount of detail. Instead, give readers enough information to understand what you did and to persuade them that what you did was adequate and that you knew what you were doing. Many journals also allow or require research protocols or more detailed information on the methods to be included in online supplemental information.
The methods section is usually the easiest part of the article to write simply because you describe what you did in your research. For this reason, many authors write this section first.
The methods section may need to address several topics, often under their own subheadings:
- research design;
- location and setting of the study;
- patient or sample selection;
- group assignment or case definitions;
- the intervention or exposure studied;
- how variables were defined and measured;
- statistical methods.
Here, I briefly describe what to consider when describing the study design, the sample selection, and the variables and how they were measured. (See “Reporting Public Health Research Methods” elsewhere in this issue of the Journal.)
The purpose of the study
The purpose of the study must be clear. The purpose is never “to study”, “to evaluate”, “to compare” or “to investigate”, which are actions, not purposes. Instead, the purpose is “to determine”, “to verify”, “to describe” or “to select”, which refer specifically to what you sought to accomplish with the research (5).
The reasons for doing the study must also be strong. Phrases such as “little is known about” (what I call “LIKA” studies (5) or that “such-and-such is not well described” do not justify a study. Neither does the phrase, “Similar studies have not been conducted here.” Consider this original and revised introduction:
- Original: little is known about the outcomes in preterm infants with heart defects and the factors associated with surgery and survival.
- Revised: the indications for offering either corrective or palliative surgery to preterm infants with heart defects are largely unknown. We conducted this study to help inform this decision.
In the original introduction, the authors used the LIKA justification, which hides the fact that the study actually addressed a compelling problem: what should surgeons tell the parents of premature infants born with severe heart defects? Which conditions are associated with survival and so would favor corrective surgery, and which are associated with death and so would favor palliative care?
Study design
To identify valid biological relationships, studies have to be as free as possible from error (random error, mistakes, or incomplete processes), confounding (the failure to rule out alternative explanations), and bias (systematic as opposed to random error). There are hundreds of sources of error, confounding, and bias. Minimizing these sources is the purpose of specific research design features and activities. For example, measurement error can be minimized by duplicating a measurement; sampling error (error caused by studying only a sample of a population) can be minimized by using adequate sample sizes and assessed with confidence intervals; and random error can be assessed with P values. Selection bias can be minimized with random assignment, and expectation and surveillance biases can be minimized with blinding. Finally, confounding can be minimized by studying the literature on the problem to identify important covariates and relationships in advance. You will not be able to tell that “having a best friend at work” predicts workplace performance if you do not ask the question in the first place (18).
Identify the study design (Figure 1). Follow any evidence-based reporting standards that are associated with your research. In particular, the STROBE guidelines (Strengthening the Reporting of Observational studies in Epidemiology) are often used in public health and epidemiological research (20). These guidelines are a minimum set of standards for reporting case-control, cross-sectional, and cohort studies. Other common study types in public health include case reports (21), outbreaks (22), analyses of clinical registries and databases (23), cluster randomized trials (24), systematic reviews (25), and economic evaluations (26). These and other reporting guidelines can be found on the EQUATOR-NET web site: http://www.equator-network.org.

Give the dates of the data collection period and say why you choose those dates (27). Reporting the dates places your study in time relative to other, possibly relevant events and can provide additional information, such as how long it took to reach the targeted sample size. When reading studies, you should look for these dates. A study published in 2018 with a data collection period from 2010 to 2012 might be outdated or it might be a solid study with a 5-year follow up.
Sample selection
The nature and size of the sample and how it was selected are critical in any research study. Ideally, the sample will be representative: it will reflect exactly the characteristics of the population to which the results are to be applied. Wine drinkers pay lots of money for a bottle of wine after tasting only a sip because that sip is supposed to be representative of the taste for the rest of the bottle. Sample selection is not so simple in public health, but the principle is the same.
The sample also has to be large enough to provide estimates with enough accuracy to be useful. The outcome of a study is called the “estimated effect size”. Because studies are usually conducted with samples, the effect size can only estimate the likely effect of in intervention. How good the estimate is depends on its accuracy, which is usually expressed with a 95% confidence interval. Larger samples provide more precise estimates in the form of narrower confidence intervals. Confidence intervals, in turn, keep the interpretation of the results focused on the biological implications of the effect size and away from P values, which are essentially measures of chance as an explanation for the outcome and that have no biological meaning.
Variables and measurements
Clearly identify your explanatory and response variables. Explanatory (or independent) variables are those related to the treatment or exposure under study, and response (or dependent) variables are the outcomes or end points of interest. Primary (and secondary) end points should be specifically identified because they can determine the study design, the sample size, the order in which the results are presented, and the interpretation of the study.
Science depends on measurement. Thus, the “who, what, when, where, why, and how” of the measurements should be clearly explained. Some measurements are obvious: hypertension defined as a systolic blood pressure of 140 mm Hg or more on 3 consecutive days, for example. Others measurements may not be obvious. In some surveys, a “current smoker” is anyone who reports smoking at least one cigarette within 30 days before the survey. This definition may not be appropriate for answering some questions about smoking, but at least we know how the variable was defined.
In epidemiology, the “case definition” is the diagnostic criteria that define a disease or disability. However, different groups have proposed different case definitions for the same disease (Table 1), and case definitions for the same disease can change over time (Table 2), so it is important to specify which case definition is being used and when it was last updated.
Table 1
| United States Institute of Medicine (28) | Canadian Consensus Criteria (29) |
|---|---|
| The presence of three symptoms: | The presence of symptoms from the following six symptom categories for 6 months or longer: |
| A substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities, that persists for more than 6 months and is accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion, and is not substantially alleviated by rest, and | Fatigue, including substantial reduction in activity level |
| Post-exertional malaise and | Sleep dysfunction |
| Unrefreshing sleep | Sleep dysfunction |
| And at least one of the two following manifestations: | Pain |
| Cognitive impairment | Neurologic/cognitive manifestations; and |
| Orthostatic intolerance | Autonomic, neuroendocrine, or immune manifestations |
Table 2
| Date | Case definition |
|---|---|
| 1990 | Isolation of varicella virus from a clinical specimen |
| Significant rise in varicella antibody level by any standard serologic assay | |
| 1996 | Isolation of varicella virus from a clinical specimen |
| Significant rise in serum varicella immunoglobulin G antibody level by any standard serologic assay | |
| 1999 | Isolation of varicella virus from a clinical specimen |
| Direct fluorescent antibody | |
| Polymerase chain reaction | |
| Significant rise in serum varicella immunoglobulin G (IgG) antibody level by any standard serologic assay | |
| 2010 | Isolation of varicella virus from a clinical specimen |
| Varicella antigen detected by direct fluorescent antibody test | |
| Varicella-specific nucleic acid detected by polymerase chain reaction | |
| Significant rise in serum anti-varicella immunoglobulin G (IgG) antibody level by any standard serologic assay |
Sometimes, the outcomes of a study are judgements, rather than more objective measurements, such as blood pressure or weight. Whether a blood sample contains evidence of a pathogen is a judgment made by a microbiologist. In such cases, it is often useful to know the qualifications of the microbiologist, how many microbiologists were judging the same slides, how often they agreed on their judgments, and what they did and did not know about the slides before they reviewed them.
Statistical methods
A standard subheading in the methods is Statistical Methods. Here, you should identify the comparisons you made and the statistical analyses used to make them (27).
Conspicuously absent from many studies is the “minimum clinically important difference”, or the smallest effect size (result) that is still large enough to make a difference. If policy makers need an intervention that reduces an infection rate by 20%, a study finding only a 17% reduction means that the intervention has not met the criterion, and the authors cannot then claim that 17% was “close enough.” The minimum clinically important difference should be determined before the study to keep researchers honest when interpreting their results, as illustrated above, and because it is a key element in calculating sample size.
Improving the results: what did you find?
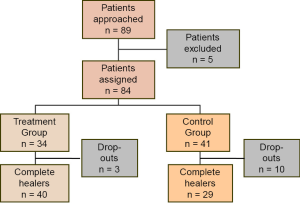
In the results, report your data, but also tell readers what happened during the study. Explain if and why the research did not go as planned. In many studies, including a visual summary or flow chart of the sample selection process can be enormously useful (Figure 2). Such flow charts are part of the STROBE (20), CONSORT (31), and PRISMA (25) reporting guidelines.
Present the results for the primary endpoint first, whether or not they are clinically important, statistically significant, or interesting. You designed the study to answer a specific question, and the results section should focus on that question. Other results can be presented, but only after the primary results have been reported.
When reporting their results, authors often put many numerical and statistical values in the text and then repeat these values in the tables and graphs. Instead, describe the most important results and refer readers to the tables or graphs for the supporting data (Figure 3). (See “Preparing Better Tables” and “Preparing Better Graphs” elsewhere in this issue of the Journal).
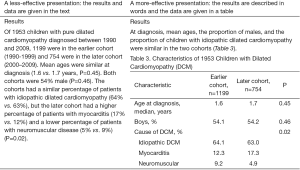
Common writing errors include using the table or figure number as the subject of the sentence and then telling readers what the title or caption already says. For example, rather than “Table 8 shows the demographic and clinical characteristics of the sample,” say “Almost 90% of patients were smokers; two-thirds of the tumors were in the glottic larynx, and one-third were in the supraglottic larynx (Table 8).” Uninformative table titles or figure captions are also unfortunately common (Figure 4). A good title or caption will identify the data and perhaps key aspects of it, such as how or when the data were collected or any qualifications needed to understand them. The goal is to allow readers to understand the data without having to refer back to the text, which is inefficient and annoying.

It is often a good idea to express results in terms of patients or people and not just the study end point. For example, instead of just reporting a mean change, tell the number of patients who improved and, if appropriate, how many crossed any thresholds in the measurement scale:
- Beck Depression scores in the treatment group decreased from a median (25th to 75th percentile) of 29 (22 to 39) to 20 (17 to 26) after 6 months. Of the 83 patients, scores improved in 61 and returned to normal (a score of 13 or less) in 5.
Improving the discussion: what does it mean?
The discussion is usually the weakest part of the article because you have to determine the meaning and implications of your results and integrate them with what else is and is not known about the topic.
I recommend writing a 7-part discussion in which you (I) summarize; (II) interpret; (III) compare; and (IV) generalize your results; (V) speculate on their implications; (VI) critique your study; and (VII) and list your conclusions (5).
Summarize the study and the main findings in a few sentences. Instead of repeating individual results, however, describe the overall findings and relate them to the reason for the study.
Interpret your results and suggest an explanation(s) for them. Describe why you think you found what you found or did not find. Here, consider only those explanations related to the study: patients declined to participate because…; survey questions were misinterpreted because…; or the results were better than expected because….
Compare your results with what else is known about the problem; that is, review the literature. Here, explain why you think you found what you found or did not find, considering what other studies have found. Did your sample include more factory workers than were included in those of other studies? Did you measure quality of life with a different instrument? Was your vaccine not the same as the one tested in other studies?
Generalize your results; say how they might relate to other patient populations or settings. If you studied adults, can the results be generalized to children? If you studied rural health care in China, can the results be generalized to rural health care in Venezuela? If your study was done in a desert climate, can the results by generalized to a tropical one? (See “How to Generalize Public Health Research Findings” elsewhere in this issue of the Journal.)
Speculate on your findings. What are their implications for public health, in your region and beyond? If needle-exchange programs for IV drug users reduce the incidence of transmittable diseases, what are the policy implications? If a new diagnostic test is far more sensitive than the current test, how many more patients may need to be treated, and will the resources be adequate for treating them? Speculation is acceptable in scientific articles, it just needs to be based on fact and logic, not suppositions and emotion, and to be called speculation, not “conclusions”.
Critique your study under the subheading “Strengths and Limitations of the Study” if possible. No one likes to admit that their study has weaknesses, but all studies have them. Peer reviewers who find limitations you did not mention, hoping no one would notice, can conclude either that you were not smart enough to recognize the limitations or that, if you were smart enough, you were trying to hide them. In either case, your credibility suffers. However, if you acknowledge the limitations, reviewers will know that you are smart enough to know they are limitations and are ethical enough not to hide them.
List your conclusions, again if possible, under the subheading “Conclusions”. Listing your conclusions will force you to be specific about what you found. Too often, specificity is lost in a general paragraph (Table 3). You may also find that your list will be longer than you thought. Remember that every conclusion needs to be supported with the data presented in the results. Also, avoid raising new topics in the conclusions.
Table 3
| Conclusions as narrative | Conclusions as a list |
|---|---|
| The literature offers little support for setting specific minimum nurse-patient ratios in acute care hospitals. Factors such as patient acuity, skill mix, nurse competence, nursing process variables, technological sophistication, and institutional support of nursing should also be considered when setting minimum ratios. Limited evidence does support probable relationships between richer nurse staffing and lower rates of needlestick injuries and nurse burnout but not for an effect on the incidence of pneumonia or urinary tract infections, although a relationship cannot be ruled out for these outcomes. The incidence of pressure ulcers, patient falls, and nosocomial infections appear to be unrelated to nurse staffing levels | We believe the evidence supports the following conclusions: |
| 1. The literature offers little support for setting specific minimum nurse-patient ratios in acute care hospitals | |
| 2. Patient acuity, skill mix, nurse competence, nursing process variables, technological sophistication, and institutional support of nursing should also be considered when setting minimum ratios | |
| 3. The evidence supports probable relationships between richer nurse staffing and (I) lower failure to rescue rates; (II) lower inpatient mortality rates; and (III) shorter hospital stays | |
| 4. Limited evidence supports probable relationships between richer nurse staffing and (I) lower rates of needlestick injuries and (II) lower rates of nurse burnout | |
| 5. The evidence neither confirms nor rules out inverse relationships between nurse staffing and the incidence of (I) pneumonia and (II) urinary tract infections | |
| 6. The evidence does not support relationships between nurse staffing and the incidence of pressure ulcers, patient falls, and nosocomial infections |
A common error is to repeat the results rather than to give their implications:
- Original conclusion: Subjects responded most appropriately to safety warnings that recommended appropriate responses and when they believed that they were able to respond as recommended. They responded least appropriately to safety warnings that did not have this information.
- Improved conclusions: Public safety warnings are more effective and are rated more useful when they recommend appropriate responses [response efficacy] and when readers believe that they are able to respond as recommended [personal efficacy]. Thus, public health officers should implement quality checks to verify that all safety warnings include information that promotes both response efficacy and personal efficacy.
Improving the references
The reference citations contain more errors than any other part of a scientific article. Authors often just do not take the time to make sure that names are spelled correctly, that journal titles are abbreviated correctly, or that page numbers are accurate. Careful authors will verify the information for each reference against the original publication. Most references can be verified by looking them up on citation databases, such as PubMed/MEDLINE (https://www.ncbi.nlm.nih.gov/pubmed/), the China Knowledge Resource Integrated (CNKI) Databases (http://en.oversea.cnki.net/kns55/default.aspx), EMBASE (Excerpta Medica dataBASE; http://www.embase.com/), HINARI (http://www.who.int/hinari/about/en/), Korean Studies Information Service System (http://kisseng.kstudy.com), and SciVerse Scopus (https://www.elsevier.com/solutions/scopus).
The journal’s guidelines for authors will tell you how to format the references. With luck, the journal will accept what are called the Uniform Requirements for Manuscripts Submitted to Biomedical Journals (https://www.nlm.nih.gov/bsd/uniform_requirements.html) (32). These requirements include a simple reference style that is accepted by several hundred life-science journals around the world. However, you should follow the journal’s reference style. If you do not, your lack of attention to the guidelines may immediately become apparent (Table 4).
Table 4
| Reference style | Example |
|---|---|
| Uniform requirements (Vancouver) (32) | Haynes RB, Mulrow CD, Huth EJ, Altman DG, Gardner MJ. More informative abstracts revisited. Ann Intern Med. 1990;113(1):69-76. Review. |
| American Medical Association (9) | Haynes RB, Mulrow CD, Huth EJ, et al. More informative abstracts revisited. Ann Intern Med. 1990 Jul 1;113(1):69-76. Review. |
| American Chemical Society (33) | Haynes, R.B.; Mulrow, C.D.; Huth, E.J.; Altman, D.G.; Gardner MJ. Ann. Intern. Med. 1990, 113:69-76. |
| American Psychological Association (34) | Haynes, R. B., Mulrow, C. D., Huth, E. J., Altman, D. G., & Gardner, M. J. (1990). More informative abstracts revisited. Annals of Internal Medicine, 113:69-76. |
| University of Chicago (35) | Haynes, R. Brian, Cynthia D. Mulrow, Edward J. Huth, Douglas G. Altman, Martin J. Gardner. 1990. More informative abstracts revisited. Ann Intern Med 113:69-76. |
Many reference lists have many errors. “Citation errors” are mistakes in the bibliographic information provided about the reference, such as the spelling of authors’ names, incomplete titles, or wrong page numbers. Minor errors are inaccuracies in the information, whereas major errors prevent the reference from being identified. Total citation error rates range up to 30%, and major error rates range up to 10% (36-41). “Quotation errors” occur when the reference is used incorrectly or inappropriately in the text. Here, a major error is when a reference cited to support a claim actually refutes it, and a minor error is when the reference does not support or is unrelated to the claim. Total quotation error rates can range up to 40%, and major error rates range up to 12% (37-39,42)
Abstracts do not contain enough information to judge the validity of the study or the accuracy of its conclusions. As a result, many journals prefer that abstracts not be cited in reference lists because they cannot provide enough evidence to support claims. In addition, major discrepancies between the abstract and full article on key points of the research are so common that the decision to cite an article should not be made only after reading the abstract (5,36,37,40).
General considerations in writing the article
You can greatly strengthen your writing by (I) not turning verbs into nouns or adjectives (creating what are called “nominalizations”) and (II) preferring the active to the passive voice in most cases (5).
Nominalizations are unfortunately common in medical writing. For example, in the sentence, “The patient was taking deep breaths”, the verb “to breathe” has been turned into the noun, “breaths”. Turning the verb into a noun means that a new verb has to be added to make the sentence complete. Usually, the new verb is weaker than the original, nominalized verb. Here, “was taking”, the new verb, has little specific information. The same sentence without the nominalization is much stronger “The patient was breathing deeply”. Here, the verb is “was breathing”, which is more specific than “was taking”.
Nominalizations are not always a problem. In the term, “advanced directive”, “directive” is a nominalization of the verb “to direct” but it is used appropriately because the term has a specific meaning. Other examples include
In the active voice, the subject of the sentence performs the action of the verb on an object: “The researchers studied the workers”. Here, the subject is the “researchers”, the verb is “studied”, and the object is “workers”. In the passive voice, which is always accompanied by a form of the verb “to be” (am, is, are, was, were, be, being, been), the object of the sentence is the grammatical subject, and the actual subject is no longer active but is instead being acted on by the verb: “The workers were studied by the researchers”. Here, the grammatical subject is the “workers”, the verb is “were studied”, and “researchers” is the object of the prepositional phrase, “by the researchers”.
The passive voice is not necessarily a problem. In fact, the passive voice is usually appropriate in the methods section because we know that the authors did the work. So, instead of saying, “We inoculated patients after we enrolled them in the study”, you can just say that “Enrolled patients were inoculated”. The problem with using the passive voice comes from combining it with nominalizations, which makes sentences longer and harder to read. In the examples below, grammatical SUBJECTS are in upper case,
- Example #1, original sentence. “During inspiration, THERE
is reversal of airflow” (7 words). - Here, “inspiration” is also a nominalization of the verb “to inspire”, but it is used correctly as a stand-alone term. “Reversal”, however, should be converted back into a verb. Also, the words “there is” contain no information and so should be replaced by a better subject and verb:
- Example #1 (better), the nominalization with the passive voice: Airflow REVERSAL
occurs during inspiration. (5 words, 30% shorter than the original). - Example #1 (preferred), without the nominalization and in the active voice: AIRFLOW
reverses during inspiration. (4 words, 40% shorter than the original). - Example #2, original sentence with two nominalizations: The study’s FAILURE
was the result of the way the principal investigator decided to submit his resignation. (17 words). - Example #2 (better), with one nominalization removed but still in the passive voice: The study’s FAILURE
was the result of the way the principal investigator resigned.(13 words; 24% shorter than the original). - Example #2 (preferred), with both nominalizations removed and in the active voice: The study
failed because of the way the principal investigator resigned. (11 words; 35% shorter than the original).
As shown in the examples, by managing just these two aspects of your writing, you can shorten your writing by up to 30% without losing information. Imagine if everything you wrote was 30% shorter and more easily understood.
Use only standard abbreviations, use them wisely (32), and resist creating your own (9). Be sure to define each abbreviation at first mention: “UA” can mean ultrasonic arteriography, urine aldosterone, uterine aspiration, unstable angina, umbilical artery, urine analysis, urine albumin, upper airway, uric acid, or even “unavailable” when used in an empty cell in a table. Also, too many closely spaced abbreviations can make reading difficult: “The NHLBI-funded CHAART study of HEU children found that in utero exposure to ARVs was associated with changes in LVEF, LV contractility, and ST/PW ratio at age 2 years” (43).
Finally, many authors have been taught to refer to themselves in an article as “the authors” because it is supposed to sound “more objective”. However, since at least 1925, the first-person pronouns, “I” and “we”, have been preferred in medical writing. According to Morris Fishbein, editor of JAMA between 1924 and 1950, “The first person singular—the naked I—is no longer thought immodest. Elaborate garments such as we and the author do not disguise a writer’s identity unless they also disguise his [sic] meaning…” (44).
Final thoughts
After you have written your article, put it aside for several days, then read it again. Have your colleagues read it closely and discuss their comments with you. Here, “enemies are better than friends”. You need someone to give you good, honest, critical comments, not someone who will tell you how well you write. Revision (literally, “to see again”) is the key to good writing. Writing is thinking, and the more you write and rewrite, the more you will think about your research and what it means. Stronger writers revise more than weaker ones do, and they let the meaning evolve as they write.
Publication is the final stage of research. You should take as much care in preparing your article as you did in conducting your research. Once published, your article will be in the literature—forever—with your name on it. Your article is an investment in your career, and you only get to publish it once, so take your time and do it right.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Public Health and Emergency for the series “Publication and Public Health”. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2017.11.06). The series “Publication and Public Health” was commissioned by the editorial office without any funding or sponsorship. TAL serves as an unpaid editorial board member of Journal of Public Health and Emergency from Jul 2017 to Jun 2019 and served as the unpaid Guest Editor of the series. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Simmons GH, Fishbein M. The Art and Practice of Medical Writing. Chicago: American Medical Association, 1925:54-5.
- Hill AB. The reasons for writing. BMJ 1965;2:870. [Crossref] [PubMed]
- Mack C. 350 years of scientific journals. J Micro/Nanolith MEMS MOEMS 2015;14(1). Available online: https://www.spiedigitallibrary.org/journals/Journal_of_MicroNanolithography_MEMS_and_MOEMS/volume-14/issue-1/010101/350-Years-of-Scientific-Journals/10.1117/1.JMM.14.1.010101.full
- DeBakey L. The Scientific Journal: Editorial Policies and Practices: Guidelines for Editors, Reviewers, and Authors. Maryland Heights, MO: C.V. Mosby, 1976.
- Lang T. How to Write, Publish, and Present in the Health Sciences: A Guide for Clinicians and Laboratory Scientists. Philadelphia: American College of Physicians, 2010.
- Heinemann M. How NOT to Write a Medical Paper: A Practical Guide. Delhi: Thieme Medical and Scientific Publishers, 2016.
- Byrne DW: Publishing Your Medical Research, 2nd edition. Alphen aan den Rijn, The Netherlands: Wolters Kluwer, 2017.
- Gastel B, Day RA. How to Write and Publish a Scientific Paper, 8th edition. Santa Barbara, CA: ABC-CLIO, 2016.
- American Medical Association. AMA Manual of Style: A Guide for Authors and Editors, 10th Edition. New York: Oxford University Press, 2007.
- Council of Science Editors. Scientific Style and Format: The CSE Manual for Authors, Editors, and Publishers, 8th edition. Chicago: University of Chicago Press, 2006.
- Relman AS. The NIH "E-Biomed" proposal—a potential threat to the evaluation and orderly dissemination of new clinical studies. N Engl J Med 1999;340:1828-9. [Crossref] [PubMed]
- Haynes RB, McKibbon KA, Walker CJ, et al. A study of the use and usefulness of on-line access to MEDLINE in clinical settings. Ann Intern Med 1990;112:78-84. [Crossref] [PubMed]
- Yoon U, Knobloch K. Assessment of reporting quality of conference abstracts in sports injury prevention according to CONSORT and STROBE criteria and their subsequent publication rate as full papers. BMC Med Res Methodol 2012;12:47. [Crossref] [PubMed]
- Ubriani R, Smith N, Katz KA. Reporting of study design in titles and abstracts of articles published in clinically oriented dermatology journals. Br J Dermatol 2007;156:557-9. [Crossref] [PubMed]
- Toma M, McAlister FA, Bialy L, et al. Transition from meeting abstract to full-length journal article for randomized controlled trials. JAMA 2006;296:653. [PubMed]
- Wong HL, Truong D, Mahamed A, et al. Quality of structured abstracts of original research articles in the British Medical Journal, the Canadian Medical Association Journal and the Journal of the American Medical Association: a 10-year follow-up study. Curr Med Res Opin 2005;21:467-73. [Crossref] [PubMed]
- Mathes J, Stevenson D. Designing Technical Reports. Indianapolis: ITT Bobbs-Merrill Educational Publishing Co., Inc., 1976.
- Gallop News. Item 10: I Have a Best Friend at Work. Bus J. May 26, 1999. Available online: http://news.gallup.com/businessjournal/511/item-10-best-friend-work.aspx
- Nissen T, Wynn R. The history of the case report: a selective review. JRSM Open 2014;5:2054270414523410 [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ 2007;85:867-72. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med 2013;2:38-43. [Crossref] [PubMed]
- Canadian Communicable Disease Reports. Outbreak reporting guide. CCDR 2015;41:4. Available online: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2015-41/ccdr-volume-41-04-april-2-2015/ccdr-volume-41-04-april-2-2015-1.html
- Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med 2015;12:e1001885 [Crossref] [PubMed]
- Campbell MK, Piaggio G, Elbourne DR, et al. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661 [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. J Clin Epidemiol 2009;62:1006-12. [Crossref] [PubMed]
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ 2013;14:367-72. [Crossref] [PubMed]
- Lang TA, Secic M. How to Report Statistics in Medicine: Annotated Guidelines for Authors, Editors, and Reviewer. 2nd ed. Philadelphia: American College of Physicians, 2006.
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations, Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining An Illness. Washington, DC: The National Academies Press, 2015.
- Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols (Canadian case definition). J Chron Fatigue 2003;11:7-115. [Crossref]
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Available online: https://wwwn.cdc.gov/nndss/conditions/varicella/
- Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med 2010;7:e1000251 [Crossref] [PubMed]
-
International Committee of Medical Journal Editors - Coghill AM, Garson LR, editors. American Chemical Society. The ACS Style Guide. 3rd ed. Washington, DC: American Chemical Society, 2006.
- The Publication Manual of the American Psychological Association. 6th ed. Washington, DC: American Psychological Association, 2010.
- Iverson C, Christiansen S, Flanagin A, et al. The Chicago Manual of Style, 16th ed. Chicago: The University of Chicago Press, 2010.
- Karabulut N. Inaccurate citations in biomedical journalism: effect on the impact factor of the American Journal of Roentgenology. AJR Am J Roentgenol 2017;208:472-4. [Crossref] [PubMed]
- Davids JR, Weigl DM, Edmonds JP, et al. Reference accuracy in peer-reviewed pediatric orthopaedic literature. J Bone Joint Surg Am 2010;92:1155-61. [Crossref] [PubMed]
- Reddy MS, Srinivas S, Sabanayagam N, et al. Accuracy of references in general surgical journals—an old problem revisited. Surgeon 2008;6:71-5. [Crossref] [PubMed]
- Al-Benna S, Rajgarhia P, Ahmed S, et al. Accuracy of references in burns journals. Burns 2009;35:677-80. [Crossref] [PubMed]
- Unver B, Senduran M, Unver Kocak F, et al. Reference accuracy in four rehabilitation journals. Clin Rehabil 2009;23:741-5. [Crossref] [PubMed]
- Raja UY, Cooper J G. How accurate are the references in Emergency Medical Journal? Emerg Med J 2006;23:625-6. [Crossref] [PubMed]
- Jergas H, Baethge C. Quotation accuracy in medical journal articles-a systematic review and meta-analysis. PeerJ 2015;3:e1364 [Crossref] [PubMed]
- Berlin L. TAC: AOITROMJA? (The Acronym Conundrum: Advancing or Impeding the Readability of Medical Journal Articles?). Available online: http://pubs.rsna.org/doi/10.1148/radiol.12121776
- Fishbein M. Medical Writing. Chicago: AMA Press, 1938:54.
Cite this article as: Lang TA. Writing a better research article. J Public Health Emerg 2017;1:88.


