
Impact of elevated urine leukocyte and bacteria count per high-power field on the in-hospital outcome of patients with liver cirrhosis
Introduction
Bacterial infection is one of the most significant complications in patients with decompensated liver cirrhosis (1). Spontaneous bacterial peritonitis (SBP) and urinary tract infection (UTI) are the most common types of bacterial infections in cirrhotic patients (2). The proportion of UTI in all bacterial infections is 20–25% (3), and the most common bacteria that cause UTI are Escherichia coli (4). Bacterial infections confer to a 4-fold increase in the mortality of cirrhosis (5). However, it remains unclear whether or not UTI increases the risk of mortality in cirrhotic patients (6).
The golden standard for diagnosis of UTI is a urine culture with significant colony counts of a single organism in a sterile manner (7). However, urine culture is not frequently used in clinical practice, especially in outpatient settings (8), for several reasons. First, a urine culture is time consuming requiring 48 hours for the growth and identification of the pathogen and additional 48–72 hours for determining its antimicrobial susceptibility. Second, a large number of cirrhotic patients with UTI are asymptomatic so that a urine culture is often not obtained (9). Third, the clinicians often use their clinical judgment rather than the standard diagnostic criteria for bacterial infections (10).
By comparison, urinalysis, microscopy, and bedside urine dipsticks are readily and rapidly available, which allows the clinicians to initiate empiric treatment for suspected UTI while awaiting urine culture results (11). Fernandez et al. also put forward that uncountable leukocytes can be used as a basis for the diagnosis of UTI, even without the urine culture result (1).
Considering that urine culture is hardly available in the clinical setting, the present study aimed to analyze the results of routine urinalysis, exploring the prevalence of abnormal urinalysis and its effect on the in-hospital outcome of cirrhotic patients.
Methods
Patients
All patients with liver cirrhosis who were consecutively admitted to our hospital between July 2010 and June 2014 and underwent urinalyses at their admission were potentially eligible, but patients with hepatocellular carcinoma and other malignancies were excluded. The study protocol was approved by the ethic committee of our hospital. The number of ethical approval was k (2017) 02. Patients’ informed consents were waived. Demographic data, clinical presentation, regular laboratory tests, Child-Pugh class, and model for end-stage liver diseases (MELD) score were also collected.
Urinalyses
A clean-catch midstream urine specimen was taken to undergo the urinalyses. Data regarding urine leukocyte and bacteria count per high-power field were collected. Their reference ranges were 0.1–4.33 and 0.1–975, respectively. We defined the results of abnormal urinalysis as a urine leukocyte count per high-power field of >4.33 and/or a urine bacteria count per high-power field of >975. If two or more urinalyses were performed, the highest urine leukocyte and bacteria count per high-power field were selected.
Statistical analyses
Continuous data were expressed as the mean ± standard deviation and the median with minimum and maximum and were compared by non-parametric Mann-Whitney-Wilcoxon tests. Categorical data were expressed as the frequency (percentage) and were compared by Chi-square test. In all comparisons, a P value of <0.05 was considered statistically significant. Risk factors associated with elevated urine leukocyte and bacteria count per high-power field were assessed by logistic regression analyses. Statistically significant variables shown in univariate analyses were entered into the multivariate analyses. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Receiver-operator characteristic (ROC) curve analyses were performed to identify the capacity of the urine leukocyte and bacteria count per high-power field in predicting the in-hospital mortality. Areas under the ROCs curve (AUROCs) with 95% CIs were calculated. The best cut-off value was selected as the sum of sensitivity and specificity was the maximum. Sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), positive predictive value (PPV), and negative predictive value (NPV) with 95% CIs were reported. SPSS statistics 17.0.0 and MedCalc version 11.4.2.0 were employed for all statistical analysis.
Results
Patients
A total of 2,067 cirrhotic patients underwent the urinalyses, of whom 2,056 had the data regarding urine leukocyte count per high-power field (Table 1) and 2,031 had the data regarding urine bacteria count per high-power field (Table 2).
Table 1
| Variables | Total (n=2,056) | Normal (n=1,526) | Abnormal (n=530) | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Pts available | Mean ± SD or frequency (percentage) | Median (range) | No. Pts available | Mean ± SD or frequency (percentage) | Median (range) | No. Pts available | Mean ± SD or frequency (percentage) | Median (range) | ||||
| Sex (male/female), n (%) | 2,056 | 1,357 (66.0%)/699 (34.0%) | 1,526 | 1,135 (74.4%)/391 (25.6%) | 530 | 222 (41.9%)/308 (58.1%) | <0.0001 | |||||
| Age (years) | 2,056 | 56.51±12.14 | 55.96 (6.20–89.23) | 1,526 | 56.09±11.92 | 55.65 (6.20–89.23) | 530 | 57.73±12.70 | 57.25 (14.37–86.84) | 0.002 | ||
| Etiology of liver diseases, n (%) | 2,056 | 1,526 | 530 | <0.0001 | ||||||||
| HBV | 592 (28.8%) | 443 (29.0%) | 149 (28.1%) | 0.688 | ||||||||
| HCV | 134 (6.5%) | 86 (5.6%) | 48 (9.1%) | 0.006 | ||||||||
| HBV + HCV | 14 (0.7%) | 8 (0.5%) | 6 (1.1%) | 0.215 | ||||||||
| Alcohol | 482 (23.4%) | 405 (26.5%) | 77 (14.5%) | <0.0001 | ||||||||
| HBV + Alcohol | 155 (7.5%) | 133 (8.7%) | 22 (4.2%) | 0.001 | ||||||||
| HCV + Alcohol | 23 (1.1%) | 17 (1.1%) | 6 (1.1%) | 0.973 | ||||||||
| HBV + HCV + Alcohol | 3 (0.1%) | 1 (0.1%) | 2 (0.4%) | 0.165 | ||||||||
| Others | 210 (10.2%) | 129 (8.5%) | 81 (15.3%) | <0.0001 | ||||||||
| Unknown | 443 (21.5%) | 304 (19.9%) | 139 (26.2%) | 0.002 | ||||||||
| Ascites, n (%) | 2,039 | 1,516 | 523 | 0.635 | ||||||||
| No | 1,034 (50.7%) | 764 (50.4%) | 270 (51.6%) | 0.628 | ||||||||
| Mild | 270 (13.2%) | 197 (13.0%) | 73 (14.0%) | 0.575 | ||||||||
| Moderate to severe | 735 (36.0%) | 555 (36.6%) | 180 (34.4%) | 0.368 | ||||||||
| HE, n (%) | 2,039 | 1,516 | 523 | 0.538 | ||||||||
| No | 1,895 (92.9%) | 1,407 (92.8%) | 488 (93.3%) | 0.702 | ||||||||
| Grade I–II | 119 (5.8%) | 88 (5.8%) | 31 (5.9%) | 0.918 | ||||||||
| Grade III–IV | 25 (1.2%) | 21 (1.4%) | 4 (0.8%) | 0.266 | ||||||||
| Laboratory tests | ||||||||||||
| RBC (1012/L) | 2,033 | 3.13±0.84 | 3.06 (0.90–6.80) | 1,512 | 3.15±0.85 | 3.10 (0.90–6.80) | 521 | 3.09±0.79 | 3 (1.10–5.90) | 0.331 | ||
| Hb (g/L) | 2,035 | 95.18±29.37 | 94 (23–218) | 1,514 | 95.50±30.12 | 94 (23–218) | 521 | 94.24±27.09 | 92 (29–176) | 0.557 | ||
| WBC (109/L) | 2,036 | 5.24±3.89 | 4.20 (0.30–46.10) | 1,515 | 5.15±3.75 | 4.10 (0.50–33) | 521 | 5.50±4.27 | 4.40 (0.30–46.10) | 0.140 | ||
| PLT (109/L) | 2,033 | 100.65±82.27 | 78 (10–1,278) | 1,512 | 100.71±83.83 | 77 (11–1,278) | 521 | 100.47±77.65 | 79 (10–545) | 0.823 | ||
| TBIL (μmol/L) | 2,027 | 40.83±65.33 | 22.10 (2–903) | 1,508 | 40.71±62.16 | 22.40 (2–679.10) | 519 | 41.19±73.85 | 21.50 (2.40–903) | 0.435 | ||
| ALB (g/L) | 1,990 | 32.19±6.87 | 32.20 (0.40–52.80) | 1,483 | 32.59±6.82 | 32.60 (0.40–52.80) | 507 | 1.03±6.87 | 30.80 (12.40–52.10) | <0.0001 | ||
| ALT (U/L) | 2,023 | 42.46±79.20 | 27 (4–1,460) | 1,505 | 42.56±80.31 | 27 (5–1,460) | 518 | 42.19±75.98 | 26 (4–1,064) | 0.607 | ||
| AST (U/L) | 2,023 | 58.53±92.46 | 37 (7–1,399) | 1,505 | 56.12±76.77 | 36 (7–1,366) | 518 | 65.53±127.38 | 37 (9–1,399) | 0.445 | ||
| Ammonia (umol/L) | 948 | 50.47±41.93 | 42 (8–480) | 707 | 50.88±42.28 | 43 (8–480) | 241 | 49.27±40.95 | 42 (8–236) | 0.443 | ||
| ALP (U/L) | 2,021 | 115.74±99.36 | 87 (12.80–980) | 1,504 | 115.01±99.09 | 87.25 (12.80–980) | 517 | 117.87±100.21 | 86 (17–889) | 0.852 | ||
| PT (second) | 1,996 | 16.36±4.54 | 15.40 (10.50–94.60) | 1,480 | 16.26±4.03 | 15.40 (10.70–62.80) | 516 | 16.65±5.77 | 15.40 (10.50–94.60) | 0.981 | ||
| APTT (second) | 1,994 | 43.17±10.34 | 41.80 (21.90–181) | 1,479 | 42.76±8.91 | 41.70 (26.90–181) | 515 | 44.34±13.60 | 42 (21.90–181) | 0.148 | ||
| INR | 1,993 | 1.35±0.56 | 1.22 (0.76–13.40) | 1,478 | 1.33±0.48 | 1.22 (0.76–7.96) | 515 | 1.39±0.76 | 1.22 (0.76–13.40) | 0.902 | ||
| GGT (U/L) | 2,019 | 115.89±202.22 | 50 (5–4,562) | 1,502 | 121.50±216.86 | 51 (6–4,562) | 517 | 99.62±150.90 | 47 (5–1,486) | 0.037 | ||
| BUN (mmol/L) | 1,990 | 7.54±6.13 | 5.81 (1.58–62.45) | 1,474 | 7.20±5.38 | 5.74 (1.58–61.88) | 516 | 8.52±7.82 | 6.07 (1.72–62.45) | 0.004 | ||
| Cr (μmol/L) | 1,990 | 82.17±106.60 | 59 (15–1,473) | 1,474 | 76.57±91.49 | 60 (21–1473) | 516 | 98.16±140 | 58.80 (15–1,069) | 0.993 | ||
| K (mmol/L) | 2,014 | 4.04±0.53 | 4 (2.26–8.28) | 1,498 | 4.05±0.52 | 4 (2.26–6.85) | 516 | 4.01±0.56 | 3.96 (2.27–8.28) | 0.015 | ||
| Na (mmol/L) | 2,014 | 138.40±4.40 | 139 (116.30–160.80) | 1,498 | 138.40±4.16 | 138.90 (121–152.40) | 516 | 138.37±5.06 | 139 (116.30–160.80) | 0.443 | ||
| Ca (mmol/L) | 983 | 2.10±0.22 | 2.10 (1.05–2.94) | 720 | 2.10±0.22 | 2.11 (1.05–2.89) | 263 | 2.09±0.21 | 2.09 (1.35–2.94) | 0.186 | ||
| Child–Pugh class, n (%) | 1,912 | 1,427 | 485 | 0.467 | ||||||||
| A | 690 (36.1%) | 515 (36.1%) | 175 (36.1%) | 0.998 | ||||||||
| B | 896 (46.9%) | 677 (47.4%) | 219 (45.2%) | 0.383 | ||||||||
| C | 326 (17.1%) | 235 (16.5%) | 91 (18.8%) | 0.246 | ||||||||
| Child–Pugh score | 1,912 | 7.54±2.04 | 7 (5–15) | 1,427 | 7.51±2 | 7 (5–15) | 485 | 7.63±2.16 | 7 (5–14) | 0.490 | ||
| MELD score | 1,936 | 7.50±7.39 | 6.14 (−9.67–54.94) | 1,433 | 7.29±6.70 | 6.17 (–8.25–42.04) | 503 | 8.12±9.07 | 6.11 (–9.67–54.94) | 0.769 | ||
| HPF–WBC (HPF) | 2,056 | 24.25±255.62 | 1.46 (0.02–8,946.90) | 1,526 | 1.25±1.02 | 0.92 (0.02–4.28) | 530 | 90.49±497.90 | 10.95 (4.43–8,946.90) | <0.0001 | ||
| HPF–Bacteria (HPF) | 2,031 | 272.53±1,107.03 | 7.13 (0.07–15,329.21) | 1,506 | 106.16±538.29 | 3.98 (0.07–9,608.11) | 525 | 749.75±1,899.42 | 42.19 (0.32–15,329.21) | <0.0001 | ||
| Death, n (%) | 2,056 | 58 (2.8%) | 1,526 | 35 (2.3%) | 530 | 23 (4.3%) | 0.014 | |||||
ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca, calcium ion; Cr, creatinine; GGT, gamma–glutamyl transpeptidase; Hb, hemoglobin; HE, hepatic encephalopathy; HPF, high–power field; INR, international normalized ratio; K, potassium; MELD, model for end stage liver disease; Na, sodium ion; PLT, platelet; PT, prothrombin time; Pts, patients; RBC, red blood cell; TBIL, total bilirubin; WBC, white blood cell.
Table 2
| Variables | Total (n=2,031) | Normal (n=1894) | Abnormal (n=137) | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Pts available | Mean ± SD or frequency (percentage) | Median (range) | No. Pts available | Mean ± SD or frequency (percentage) | Median (range) | No. Pts available | Mean ± SD or frequency (percentage) | Median (range) | ||||
| Sex (male/female), n (%) | 2,031 | 1,342 (66.1%)/ |
1,894 | 1,298 (68.5%)/596 (31.5%) | 137 | 44 (32.1%)/93 (67.9%) | <0.0001 | |||||
| Age (years) | 2,031 | 56.46±12.15 | 55.96 (6.20–89.23) | 1,894 | 56.16±12.13 | 55.66 (6.20–89.23) | 137 | 60.73±11.63 | 61.02 (30.30–85.38) | <0.0001 | ||
| Etiology of liver diseases, n (%) | 2,031 | 1,894 | 137 | 0.001 | ||||||||
| HBV | 583 (28.7%) | 546 (28.8%) | 37 (27.0%) | 0.649 | ||||||||
| HCV | 133 (6.5%) | 125 (6.6%) | 8 (5.8%) | 0.728 | ||||||||
| HBV + HCV | 14 (0.7%) | 12 (0.6%) | 2 (1.5%) | 0.243 | ||||||||
| Alcohol | 476 (23.4%) | 453 (23.9%) | 23 (16.8%) | 0.057 | ||||||||
| HBV + Alcohol | 155 (7.6%) | 153 (8.1%) | 2 (1.5%) | 0.005 | ||||||||
| HCV + Alcohol | 23 (1.1%) | 23 (1.2%) | 0 (0.0%) | 0.398 | ||||||||
| HBV + HCV + Alcohol | 3 (0.1%) | 3 (0.2%) | 0 (0.0%) | 1.000 | ||||||||
| Others | 209 (10.3%) | 189 (10.0%) | 20 (14.6%) | 0.086 | ||||||||
| Unknown | 435 (21.4%) | 390 (20.6%) | 45 (32.8%) | 0.001 | ||||||||
| Ascites, n (%) | 2,014 | 1,878 | 136 | 0.308 | ||||||||
| No | 1,021 (50.7%) | 953 (50.7%) | 68 (50.0%) | 0.867 | ||||||||
| Mild | 268 (13.3%) | 255 (13.6%) | 13 (9.6%) | 0.183 | ||||||||
| Moderate to Severe | 725 (36.0%) | 670 (35.7%) | 55 (40.4%) | 0.264 | ||||||||
| HE, n (%) | 2,014 | 1,879 | 135 | 0.146 | ||||||||
| No | 1,870 (92.9%) | 1,749 (93.1%) | 121 (89.6%) | 0.133 | ||||||||
| Grade I–II | 119 (5.9%) | 106 (5.6%) | 13 (9.6%) | 0.058 | ||||||||
| Grade III–IV | 25 (1.2%) | 24 (1.3%) | 1 (0.7%) | 1.000 | ||||||||
| Laboratory tests | ||||||||||||
| RBC (1012/L) | 2,008 | 3.13±0.84 | 3.06 (0.93–6.78) | 1,876 | 3.14±0.84 | 3.08 (0.93–6.78) | 132 | 2.98±0.81 | 2.88 (1.25–5.57) | 0.028 | ||
| Hb (g/L) | 2,008 | 95.13±29.33 | 93 (23–218) | 1,876 | 95.56±29.40 | 94 (23–218) | 132 | 88.97±27.73 | 86 (29–159) | 0.011 | ||
| WBC (109/L) | 2,008 | 5.24±3.90 | 4.20 (0.30–46.10) | 1,876 | 5.25±3.92 | 4.20 (0.50–46.10) | 132 | 5.17±3.72 | 4.20 (0.30–26.30) | 0.661 | ||
| PLT (109/L) | 2,008 | 100.70±82.38 | 77.50 (10–1278) | 1,876 | 101.10±83.23 | 78 (10–1278) | 132 | 94.99±69.23 | 74.50 (13–443) | 0.641 | ||
| TBIL (μmol/L) | 2,003 | 40.56±64.85 | 22 (2–903) | 1,869 | 39.99±64.12 | 22.20 (2–903) | 134 | 48.58±74.14 | 20.70 (5.30–383.20) | 0.827 | ||
| ALB (g/L) | 1,967 | 32.22±6.84 | 32.20 (0.40–52.80) | 1,837 | 32.33±6.85 | 32.40 (0.40–52.80) | 130 | 30.60±6.48 | 30.50 (15.20–47.30) | 0.004 | ||
| ALT (U/L) | 1,999 | 42.49±79.62 | 27 (4–1,460) | 1,865 | 42.17±77.74 | 27 (4–1460) | 134 | 46.94±102.54 | 24 (7–748) | 0.186 | ||
| AST (U/L) | 1,999 | 58.35±92.63 | 37 (7–1,399) | 1,865 | 57.04±82.75 | 37 (7–1366) | 134 | 76.56±180.47 | 36 (10–1,399) | 0.801 | ||
| Ammonia (μmol/L) | 1,999 | 58.35±92.63 | 37 (7–1,399) | 1,865 | 57.04±82.75 | 37 (7–1366) | 134 | 76.56±180.47 | 36 (10–1,399) | 0.801 | ||
| ALP (U/L) | 903 | 52.05±42.15 | 43 (9–480) | 847 | 51.68±42.05 | 43 (9–480) | 56 | 57.70±43.63 | 48 (9–236) | 0.241 | ||
| PT (second) | 1,973 | 16.35±4.51 | 15.40 (10.50–94.60) | 1,838 | 16.35±4.56 | 15.40 (10.50–94.60) | 135 | 16.39±3.76 | 15.50 (11.50–38.90) | 0.598 | ||
| APTT (second) | 1,968 | 42.96±8.87 | 41.80 |
1,834 | 42.92±8.84 | 41.80 (21.90–152.70) | 134 | 43.53±9.31 | 41.50 (28–81.70) | 0.610 | ||
| INR | 1,970 | 1.35±0.56 | 1.22 (0.76–13.40) | 1,835 | 1.35±0.57 | 1.22 (0.76–13.40) | 135 | 1.34±0.43 | 1.23 (0.84–4.13) | 0.615 | ||
| GGT (U/L) | 1,994 | 115.94±203.16 | 50 (5–4,562) | 1,860 | 117.91±208.15 | 50 (5–4,562) | 134 | 88.59±110.11 | 51.50 (8–709) | 0.348 | ||
| BUN (mmol/L) | 1,967 | 7.55±6.14 | 5.82 (1.58–62.45) | 1,835 | 7.48±6.05 | 5.80 (1.58–62.45) | 132 | 8.53±7.28 | 6.25 (1.95–44.34) | 0.035 | ||
| Cr (μmol/L) | 1,967 | 81.72±105.07 | 59 (15–1,473) | 1,835 | 81.35±103.68 | 60 (15–1,473) | 132 | 86.99±123.10 | 56 (29–919) | 0.301 | ||
| K (mmol/L) | 1,992 | 4.04±0.53 | 4 (2.26–8.28) | 1,858 | 4.04±0.53 | 4 (2.27–8.28) | 134 | 3.96±0.47 | 3.96 (2.26–5.38) | 0.075 | ||
| Na (mmol/L) | 1,992 | 138.38±4.56 | 139 (83–160.80) | 1,858 | 138.42±4.50 | 139 (83–160.80) | 134 | 137.85±5.37 | 138.90 (116.30–148) | 0.484 | ||
| Ca (mmol/L) | 970 | 2.10±0.22 | 2.10 (1.05–2.94) | 895 | 2.09±0.22 | 2.10 (1.05–2.94) | 75 | 2.12±0.20 | 2.10 (1.76–2.62) | 0.341 | ||
| Child–Pugh class, n (%) | 1,891 | 1,764 | 127 | 0.516 | ||||||||
| A | 685 (36.2%) | 643 (36.5%) | 42 (33.1%) | 0.444 | ||||||||
| B | 884 (46.7%) | 825 (46.8%) | 59 (46.5%) | 0.946 | ||||||||
| C | 322 (17.0%) | 296 (16.8%) | 26 (20.5%) | 0.285 | ||||||||
| Child–Pugh score | 1,891 | 7.54±2.04 | 7 (5–15) | 1,764 | 7.52±2.03 | 7 (5–15) | 127 | 7.83±2.18 | 8 (5–14) | 0.149 | ||
| MELD score | 1,915 | 7.46±7.35 | 6.11 (−9.67–54.94) | 1,787 | 7.43±7.31 | 6.12 (–9.67–54.94) | 128 | 7.90±7.93 | 6.11 (–4.56–35.30) | 0.795 | ||
| HPF–WBC (HPF) | 2,031 | 24.50±257.18 | 1.44 (0.02–8,946.90) | 1,894 | 10.69±74.88 | 1.35 (0.02–2,417.09) | 137 | 215.38±932.64 | 14.09 (0.20–8,946.90) | <0.0001 | ||
| HPF–Bacteria (HPF) | 2,031 | 272.53±1,107.03 | 7.13 (0.07–15,329.21) | 1,894 | 56.18±139.60 | 5.58 (0.07–965.90) | 137 | 3,263.51±2,890.99 | 2,213.55 (992.68–15,329.21) | <0.0001 | ||
| Death, n (%) | 2,031 | 57 (2.8%) | 1,894 | 47 (2.5%) | 137 | 10 (7.3%) | 0.004 | |||||
ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca, calcium ion; Cr, creatinine; GGT, gamma–glutamyl transpeptidase; Hb, hemoglobin; HE, hepatic encephalopathy; HPF, high–power field; INR, international normalized ratio; K, potassium; MELD, model for end stage liver disease; Na, sodium ion; PLT, platelet; PT, prothrombin time; Pts, patients; RBC, red blood cell; TBIL, total bilirubin; WBC, white blood cell.
Urine leukocyte count per high-power field
The prevalence of elevated urine leukocyte count per high-power field was 25.8% (530/2,056). Elevated urine leukocyte count per high-power field was significantly associated with female, etiology of liver diseases, older age, higher blood urea nitrogen (BUN), and lower albumin (ALB), potassium, and gamma-glutamyl transpeptidase (GGT) (Table 1). Logistic regression multivariate analysis demonstrated that female (P<0.0001, OR =4.71), ALB (P<0.0001, OR =0.97), and BUN (P<0.0001, OR =1.05) were independently associated with elevated urine leukocyte count per high-power field (Table 3).
Table 3
| Variables | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |||
| HCV as an etiology of liver diseases | ||||||
| Sex | 4.03 (3.27–4.96) | <0.0001 | 4.76 (3.77–6.00) | <0.0001 | ||
| Age | 1.01 (1.00–1.02) | 0.007 | 0.99 (0.98–1.00) | 0.071 | ||
| HCV | 0.60 (0.42–0.87) | 0.006 | 0.77 (0.51–1.15) | 0.195 | ||
| ALB | 0.97 (0.95–0.98) | <0.0001 | 0.97 (0.95–0.98) | <0.0001 | ||
| GGT | 0.99 (0.99–1.00) | 0.032 | 1.00 (0.99–1.00) | 0.311 | ||
| BUN | 1.03 (1.02–1.05) | <0.0001 | 1.05 (1.03–1.06) | <0.0001 | ||
| K | 0.85 (0.70–1.02) | 0.086 | ||||
| Alcohol as an etiology of liver diseases | ||||||
| Sex | 4.03 (3.27–4.96) | <0.0001 | 4.64 (3.62–5.95) | <0.0001 | ||
| Age | 1.01 (1.00–1.02) | 0.007 | 0.99 (0.98–1.00) | 0.085 | ||
| Alcohol | 2.13 (1.63–2.78) | <0.0001 | 1.13 (0.83–1.55) | 0.436 | ||
| ALB | 0.97 (0.95–0.98) | <0.0001 | 0.97 (0.95–0.98) | <0.0001 | ||
| GGT | 0.99 (0.99–1.00) | 0.032 | 1.00 (0.99–1.00) | 0.322 | ||
| BUN | 1.03 (1.02–1.05) | <0.0001 | 1.05 (1.03–1.06) | <0.0001 | ||
| K | 0.85 (0.70–1.02) | 0.086 | ||||
| HBV + Alcohol as an etiology of liver diseases | ||||||
| Sex | 4.03 (3.27–4.96) | <0.0001 | 4.74 (3.75–6.01) | <0.0001 | ||
| Age | 1.01 (1.00–1.02) | 0.007 | 0.99 (0.98–1.00) | 0.080 | ||
| HBV + Alcohol | 2.21 (1.39–3.50) | 0.001 | 1.16 (0.71–1.89) | 0.563 | ||
| ALB | 0.97 (0.95–0.98) | <0.0001 | 0.97 (0.95–0.98) | <0.0001 | ||
| GGT | 0.99 (0.99–1.00) | 0.032 | 1.00 (0.99–1.00) | 0.260 | ||
| BUN | 1.03 (1.02–1.05) | <0.0001 | 1.05 (1.03–1.06) | <0.0001 | ||
| K | 0.85 (0.70–1.02) | 0.086 | ||||
ALB, albumin; BUN, blood urea nitrogen; GGT, gamma–glutamyl transpeptidase; HBV, hepatitis B virus; HCV, hepatitis C virus; K, potassium.
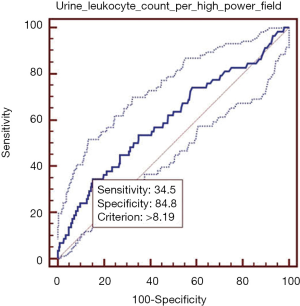
Elevated urine leukocyte count per high-power field was significantly associated with higher in-hospital mortality. In ROC analysis, the AUROC of urine leukocyte count per high-power field for predicting the in-hospital death was 0.600 (95% CI: 0.579–0.622, P=0.015) (Figure 1). The best cut-off value of urine leukocyte count per high-power field was 8.19, with a sensitivity of 34.5% (95% CI: 22.5–48.1%) and a specificity of 84.8% (95% CI: 83.1–86.3%). PLR and NLR were 2.27 (95% CI: 1.6–3.2) and 0.77 (95% CI: 0.6–1.0), respectively. PPV and NPV were 6.2% (95% CI: 3.8–9.4%) and 97.8% (95% CI: 97.0–98.4%), respectively.
Urine bacteria count per high-power field
The prevalence of elevated urine bacteria count per high-power field was 6.7% (137/2,031). Elevated urine bacteria count per high-power field was significantly associated with female, etiology of liver diseases, higher age and BUN, and lower red blood cells, hemoglobin, and ALB (Table 2). Logistic regression multivariate analysis demonstrated that female (P<0.0001, OR =3.73), age (P=0.027, OR =1.02), and ALB (P=0.019, OR =0.96) were independently associated with elevated urine bacteria count per high-power field (Table 4).
Table 4
| Variables | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Sex | 4.60 (3.18–6.67) | <0.0001 | 3.73 (2.50–5.58) | <0.0001 | |
| Age | 1.03 (1.02–1.05) | <0.0001 | 1.02 (1.00–1.03) | 0.027 | |
| HBV + Alcohol | 5.93 (1.45–24.19) | 0.013 | 2.34 (0.56–9.82) | 0.245 | |
| ALB | 0.96 (0.94–0.99) | 0.005 | 0.96 (0.93–0.99) | 0.019 | |
| RBC | 0.79 (0.64–0.99) | 0.037 | 1.33 (0.81–2.19) | 0.262 | |
| Hb | 0.99 (0.99–1.00) | 0.013 | 0.99 (0.98–1.00) | 0.095 | |
| BUN | 1.02 (0.99–1.05) | 0.062 | |||
ALB, albumin; BUN, blood urea nitrogen; Hb, hemoglobin; HBV, hepatitis B virus; RBC, red blood cell.
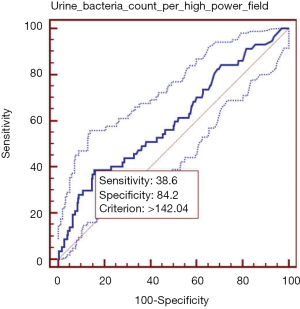
Elevated urine bacteria count per high-power field was significantly associated with higher in-hospital mortality. In ROC analysis, the AUROC of urine bacteria count per high-power field for predicting the in-hospital death was 0.600 (95% CI: 0.578–0.622, P=0.014) (Figure 2). The best cut-off value of urine bacteria count per high-power field was 142.04, with a sensitivity of 38.6% (95% CI: 26.0–52.4%) and a specificity of 84.19% (95% CI: 82.5–85.8%). PLR and NLR were 2.44 (95% CI: 1.8–3.4) and 0.73 (95% CI: 0.6–0.9), respectively. PPV and NPV were 6.6% (95% CI: 4.2–9.8%) and 97.9% (95% CI: 97.1–98.6%), respectively.
Discussion
We here demonstrate on a large single-center cohort a high prevalence of elevated urine leukocyte and bacteria count per high-power field of 25.8% and 6.7%, respectively. In addition, these simple screening tests predicted the in-hospital death with a moderate diagnostic accuracy. Although their sensitivity was low, they showed an excellent specificity of >80%.
Urinalysis represents a non-invasive, technically simple, and economic screening tool (12). Lee et al. suggested that the presence of at least 5 urine leukocyte counts per high-power field from urine specimen should be pyuria, which was observed in 67% (165/247) of patients(13). Cantey et al. pointed that urinalysis was positive if >10 leukocytes per oil immersion field were seen (14). Gieteling et al. indicated that the presence of ≥10 leucocytes per high-power field should be helpful for a diagnosis of UTI (15). Thus, urinalysis, such as urine leukocyte and bacteria count per high-power field, may be helpful to establish a rapid diagnosis of UTI in the absence of urine culture. If possible, empirical antibiotic treatment can be rapidly guided by abnormal urinalyses.
The prevalence of UTI in liver cirrhosis patients is 20–25%, which is confirmed on our cohort (3). We included a large number of cirrhotic patients over a 4-year period of time. Therefore, our data may be more generalizable.
The association between UTI and severity of liver dysfunction remained controversial. Previous studies demonstrated that the occurrence of UTI was associated with Child-Pugh score (9,16) and ascites (6,17). By contrast, our and Amato et al.’s (18) studies demonstrated that the prevalence of UTI was not significantly associated with liver disease severity. This discrepancy might be explained by the heterogeneity in the sample size, the patient characteristics and the use of diuretics.
It is generally accepted that patients with cirrhosis are susceptible to the development of infectious diseases and that bacterial infection may aggravate the deterioration of patients’ conditions, even leading them to death (19). Our study found a significant association between abnormal urinalysis (i.e., elevated urine leukocyte and/or bacteria count per high-power field count) and in-hospital mortality of cirrhotic patients. Indeed, in our patients, 23 of 58 deaths had an elevated urine leukocyte count per high-power field. Similarly, a retrospective observational cohort study also demonstrated an association of UTI with increased short-term mortality in patients with advanced cirrhosis (6). Despite the direct contribution of UTI to the risk of death in cirrhotic patients remained uncertain, abnormal urinalysis might be a predictor of worse prognosis. Evidence suggested that 42% of advanced liver disease patients with UTI have systemic inflammatory response syndrome (20) and that UTI is a strong reason for progressive renal failure in cirrhosis (21).
Our study had some limitations. First, we recorded urine leukocyte count per high-power field of >4.33. Abnormal urinalysis is not exactly equal to positive urine culture (22). Thus, our study could not accurately identify the diagnosis of UTI. Second, there was a potential risk of urine specimens’ contamination. Third, the symptoms related to UTI (i.e., fever, urinary frequency, and urinary urgency) were missing.
In conclusion, an elevated urine leukocyte and/or bacteria count per high-power field may be an adjuvant diagnostic criterion for UTI and should be a predictor for the in-hospital death in patients with liver cirrhosis. In future, some novel noninvasive screening tools for liver damage, such as M30 levels (23), or for liver fibrosis, such as transient elastography (24), should be combined with UTI to further evaluate the disease progression and outcome of liver cirrhosis.
Acknowledgments
Funding: This study was partially supported by the grants from the Natural Science Foundation of Liaoning Province (no. 2015020409) and China Postdoctoral Science Foundation (2015M582886) for Dr Xingshun Qi.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2017.08.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).The study protocol was approved by the ethic committee of our hospital. The number of ethical approval was k (2017) 02. Patients’ informed consents were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fernandez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002;35:140-8. [Crossref] [PubMed]
- Bajaj JS, O'Leary JG, Wong F, et al. Bacterial infections in end-stage liver disease: current challenges and future directions. Gut 2012;61:1219-25. [Crossref] [PubMed]
- Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol 2016;8:307-21. [Crossref] [PubMed]
- Xu Y, Wang JB, Wang S. Zhonghua Gan Zang Bing Za Zhi 2016;24:478-80. [Association between chronic urinary tract infection and primary biliary cirrhosis]. [PubMed]
- Arvaniti V, D'Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010;139:1246-56, 1256.e1-5.
- Reuken PA, Stallmach A, Bruns T. Mortality after urinary tract infections in patients with advanced cirrhosis - Relevance of acute kidney injury and comorbidities. Liver Int 2013;33:220-30. [Crossref] [PubMed]
- Yamasaki Y, Uemura O, Nagai T, et al. Pitfalls of diagnosing urinary tract infection in infants and young children. Pediatr Int 2017; [Crossref] [PubMed]
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002;113:5S-13S. [Crossref] [PubMed]
- Borzio M, Salerno F, Piantoni L, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis 2001;33:41-8. [Crossref] [PubMed]
- Caterino JM, Leininger R, Kline DM, et al. Accuracy of Current Diagnostic Criteria for Acute Bacterial Infection in Older Adults in the Emergency Department. J Am Geriatr Soc 2017; [Crossref] [PubMed]
- Felt JR, Yurkovich C, Garshott DM, et al. The Utility of Real-Time Quantitative Polymerase Chain Reaction Genotype Detection in the Diagnosis of Urinary Tract Infections in Children. Clin Pediatr (Phila) 2017;9922817706144 [PubMed]
- Sidler D, Huynh-Do U. Urinalysis in the 21st century: anything but obsolete! Praxis (Bern 1994) 2015;104:349-52.
- Lee JR, Bang H, Dadhania D, et al. Independent risk factors for urinary tract infection and for subsequent bacteremia or acute cellular rejection: a single-center report of 1166 kidney allograft recipients. Transplantation 2013;96:732-8. [Crossref] [PubMed]
- Cantey JB, Gaviria-Agudelo C, McElvania TeKippe E, et al. Lack of clinical utility of urine gram stain for suspected urinary tract infection in pediatric patients. J Clin Microbiol 2015;53:1282-5. [Crossref] [PubMed]
- Gieteling E, van de Leur JJ, Stegeman CA, et al. Accurate and fast diagnostic algorithm for febrile urinary tract infections in humans. Neth J Med 2014;72:356-62. [PubMed]
- Cadranel JF, Denis J, Pauwels A, et al. Prevalence and risk factors of bacteriuria in cirrhotic patients: a prospective case-control multicenter study in 244 patients. J Hepatol 1999;31:464-8. [Crossref] [PubMed]
- Bercoff E, Dechelotte P, Weber J, et al. Urinary tract infection in cirrhotic patients, a urodynamic explanation. Lancet 1985;1:987. [Crossref] [PubMed]
- Amato A, Precone DF, Carannante N, et al. Infez Med 2005;13:103-8. [Prevalence and risk factors for bacteriuria in patients with cirrhosis]. [PubMed]
- Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol 2007;102:1510-7. [Crossref] [PubMed]
- Cazzaniga M, Dionigi E, Gobbo G, et al. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol 2009;51:475-82. [Crossref] [PubMed]
- Kim JH, Lee JS, Lee SH, et al. Renal Dysfunction Induced by Bacterial Infection other than Spontaneous Bacterial Peritonitis in Patients with Cirrhosis: Incidence and Risk Factor. Gut Liver 2009;3:292-7. [Crossref] [PubMed]
- Waseem M, Chen J, Paudel G, et al. Can a simple urinalysis predict the causative agent and the antibiotic sensitivities? Pediatr Emerg Care 2014;30:244-7. [Crossref] [PubMed]
- Mueller S, Nahon P, Rausch V, et al. Caspase-cleaved keratin-18 fragments increase during alcohol withdrawal and predict liver-related death in patients with alcoholic liver disease. Hepatology 2017;66:96-107. [Crossref] [PubMed]
- Mueller S, Sandrin L. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepat Med 2010;2:49-67. [Crossref] [PubMed]
Cite this article as: Han D, Wang R, Yu Y, Yang SS, Mueller S, Romeiro FG, Song T, Deng H, Li J, Peng Z, Li Y, Guo X, Qi X. Impact of elevated urine leukocyte and bacteria count per high-power field on the in-hospital outcome of patients with liver cirrhosis. J Public Health Emerg 2017;1:73.


