Exposure to environmental endocrine disruptors and human health
Introduction
Endocrine-disrupting compound is named by the U.S. Environmental Protection Agency (EPA) as “an agent that interferes with the synthesis, secretion, transport, binding or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development and/or behavior” (1). Many chemicals have been identified as endocrine disruptors and they have been active for the past decade. These chemicals are related to many chronic diseases like breast cancer, ovarian problems, thyroid eruptions, testicular carcinoma, diabetes, reproductive abnormalities, nerve damage, obesity and many homeostatic imbalance (2).
Environmental endocrine disruptors (EEDs) are compounds altering the physiological function of endocrine system in both human and wild life. They can impact endocrine system by targeting different levels of the hypothalamic-pituitary-thyroid/gonad/adrenal axes, inhibiting or stimulating the production of hormones, or changing the way hormones travel through the body, the effects can range from hormone receptors to hormone synthesis or metabolism, so the EEDs can have negative health implications on human (3). Over the years, scientists, physicians and governments are paying more and more attention to potential links between exposure to EEDs and certain endocrine-related diseases, resulting form that exposure to many EEDs is now ubiquitous, coupled with suggested trends for increased rates of some endocrine-related diseases.
This review provides a brief introduction to some EEDs such as PAHs, contemporary use pesticides, bisphenol A (BPA), phthalates, heavy metals, representing a small number of all known or suspected EEDs. Besides this, we also give a summarize of EEDs for adverse impacts on human development (reproductive tract development, thyroid function, effect on nervous system, obesity and diabetes), guidance and recommendations for future research.
Classification of EEDs
EEDs can be grouped according to different observations. In this paper, we categorized EEDs into five groups as following.
PAHs
PAHs are lipophilic chemicals with long half-lives that include polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and other brominated flame retardants (BFRs). PBDEs are now being widely used in carpet backing, furniture and other consumer products that we use regular in our daily life (4). Exposure to it can also occur through dietary sources, but contact with house dust may account for the primary source. Fortunately, use of PBDEs have already been prohibited or are currently being phased out in many countries. PCBs are widely used as transformer, hydraulic fluids and additives existing in paints and other building materials (5). PCBs exposure from building materials is possible, but continued exposures to PCBs can occur primarily through the diet (meat, higher trophic level fish etc.). BFRs and other flame retardants can be found in different products range from electronics, textiles, computers, foam furniture and so on (6). Since BFRs are not bound to the products and are easily released into the environment, these products are considered as potential source of endocrine disruptors. However, they have been banned in many countries, but BFRs are still being considered as pollution source since they persist in the environment for longer period because of their longer half-life (7).
There are scientific researchers suggesting that PAHs are associated with many adverse health effects such as thyroid effects (8), obesity (9), diabetes (10), reproductive problems (11,12) and endocrine-related cancers (13).
Contemporary use pesticides
Pesticides are composed of insecticides, fungicides, herbicides, rodenticides, etc. The most human research has been conducted for insecticides and the common types of insecticides are organophosphates, organochlorine, pyrethroids and carbamates. DDT which belongs to the pyrethroids was once used popular as a pesticide in agricultural sectors, household and public places, but it was banned few years back because of its hazardous nature. However, the compound is still in use in some countries. Organophosphorus pesticides (OPs) are the most commonly used pesticide and chlorpyrifos is a unique example. The insecticide is regarded as safe alternatives to arsenic-based pesticides after 1940s and is used in both household and agriculture field to control pests such as cockroaches, flies, mosquitoes, termites. However, it is confirmed from studies that the compound is highly toxic since it has a huge effect on nervous systems (14), some of heavily used organochlorine pesticides (OCPs) were banned in industrialized nations in the 1970s after reports of the negative effect on human, but the use of DDT still occurs in some developing countries as an effective method against vector-borne illnesses.
Today nearly all pesticides we used are designed to disintegrate after some hours or days (just called “non-persistent”). But it has been shown that many non-persistent pesticides can remain for years after applied in homes where they are protected from sunlight, moisture and other degradation mechanisms (15). Pesticides are usually designed to be highly sensitive toward reproductive and neural systems of the organisms, but due to the similarity with human physiological function, these chemicals can also be harm to normal human body (16), so they are known neurovirulence, especially in acute high-dose situations. DDT can interfere with thyroid, insulin, reproductive and neuroendocrine systems (17-20), which has made it one of the most potential candidates as endocrine disruptors.
BPA
BPA is a high production chemical that is used frequently in polycarbonate-plastic based containers, thermal paper and the lining of canned food. Because of the negative effects on human being, the compound is banned from using in baby bottles. But it is still found in many containers, especially in the epoxy based lining of canned foods which are used for soups, fruit jam etc. and in certain construction materials (21). Although the lining is used to give protection from pathogens, it is in direct contact with food and finally grasp the opportunity entering food and then into human bodies. Exposure to BPA occurs mainly through diet, and measurable levels of BPA can be found in most people (5).
A diverse variety of developmental problems following exposure to BPA such as abnormal reproductive organ and neurobehavioral effects have been reported in some animal studies (22-24). However, human studies remain limited, a little ones have been suggested relationship between BPA exposure and reproductive endpoints, altered thyroid hormones, cardiovascular disease and diabetes (25-28).
Phthalic acid esters (PAEs)
PAEs can be used as solubilizing or stabilizing agents, and also service as plasticizer to make plastics more flexible, they can be found in a great deal of products. Due to their widespread use, phthalate-metabolites can be measured in urinary of virtually everyone. High molecular weight phthalates exist in flexible PVC commonly induced in food packaging, home furnishings and other materials. Low molecular weight phthalates can be found in personal care products, certain dietary supplements and other consumer goods. Unfortunately, elevated exposure to phthalates has been reported among infants as a result of phthalate-containing medical equipment or personal care products (29).
Several phthalates have demonstrated adverse impact on reproductive development and numerous other endpoints at high doses in rodents (30,31). In human studies, phthalates have been associated with abnormal in sex steroid, thyroid hormone levels (32), insulin resistance (33), poor sperm quality (34) and obesity (35), they have also something to do with type 2 diabetes in human populations (36).
Other chemicals
There are many other types of EEDs to which extensive approach of human exposure has been involved. Heavy metals are public health concern recently because they can persist in the environment for long time and some metals such as cadmium, lead and arsenic have biological half-lives of longer than 10 years (37). Cadmium, arsenic, mercury and lead included in heavy metals are classified as EEDs and thought to have estrogenic activity (38). Lead is a natural compound used in mining, refining, gasoline, lead-acid batteries, jewellery, children’s products and many other products. It is worth mentioning that children are the potential candidates of lead poisoning, due to their high ingest quantity but do not have a fully developed blood-brain barrier (39). A large body of evidence shows that heavy metals, such as lead, cadmium, arsenic and mercury may impact endocrine function in addition to their other modes of toxicity (40-43).
There is also a growing list of other emerging EEDs including triclosan, parabens, perchlorate and fluorinated organic compounds such as perfluorooctane sulfonate (PFOS). There are researchers suggesting that many of these emerging compounds may be associated with endocrine endpoints in animals and humans (44-47).
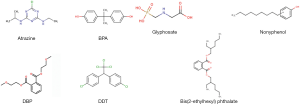
Humans can be exposed to EEDs through dietary, occupations or environmental exposure (water, soil and air). Table 1 lists some common EEDs and their uses. Because of the diverse mechanism of EEDs in human body, it is very difficult to establish a relationship between EEDs if just considering the structural features of them (some of the common structures of EEDs are given in Figure 1). Actually, it is sometimes the metabolites of EEDs more hazardous than the compound itself. The rest of this article will pay attention to developmental endpoints associated with those EEDs and their metabolites introduced in previous paragraphs.
Table 1
| Common EEDs | Uses in our daily life |
|---|---|
| Polybrominated diphenyl ethers (PBDEs) | Carpet backing, furniture, dietary sources |
| Polychlorinated biphenyls (PCBs) | Transformer, hydraulic fluids, additives, building materials, diet (meat, higher trophic level fish etc.) |
| Brominated flame retardants (BFRs) | Electronics, textiles, computers, foam furniture |
| DDT, chlorpyrifos, atrazine, glyphosate | Pesticides |
| Bisphenol A (BPA) | Polycarbonate-plastic based containers, thermal paper, epoxy based lining of canned food, certain construction materials |
| Phthalates | Plastics, food packaging, home furnishings, personal care products, dietary supplements, medical equipment |
| Lead | Smelting, mining, refining, leaded petrol (gasoline), lead-acid batteries, paints, jewellery, children’s products |
| Triclosan | Antibacterials |
| Perfluorochemicals | Textiles and clothing |
EEDs, environmental endocrine disruptors.
Developmental disease endpoints related to EEDs
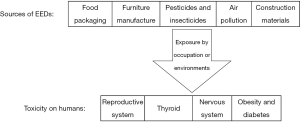
EEDs may alter the homeostatic system through environmental exposure. Different studies (animal, clinical observations and epidemiological studies) have indicated the potential role of endocrine disruptors in affecting reproductive and nervous systems, thyroid, metabolism, breast, lung and causing obesity. Some effects of EEDs on human health are discussed below. Figure 2 shows the routes of EEDs releasing into the environment and their toxic effects on humans.
Reproductive tract development
EEDs damage normal physiological reactions about the reproductive system. It is known that female sexual differentiation occurs independent of estrogen and androgen, which largely mediate male differentiation. There are some effects of EEDs on male and female reproductive system which are as follows.
Male reproduction and development
There is evidence that anomalies of the male reproductive tract such as sperm anomalies, hypospadias and ectopic testes may have increased in some countries recently (48). These diseases are possibly caused by perinatal exposure to EEDs during sensitive stages of the developing fetus (49).
EEDs reduce number as well as quality of sperms, along with an increase in occurrence of testicular, prostate and breast cancer (50). Similarly, low sperm count due to toxicity of environmental pollutants has been reported (51-53). Testicular dysgenesis syndrome (TDS) is one of the main illnesses being associated with some environmental endocrine pollutants (54). It represents a series of reproductive disorders disturbing gonadal development (hypospadias, cryptorchidism and smaller reproductive organs) (55). TDS can effect on human as a reduction in semen quality, infertility and increasing risk for testicular cancer as they growing older.
Several studies that have been reviewed previously have assessed relationships between EEDs and male genital defects. A relationship between prenatal PBDE exposure and congenital cryptorchidism was reported in Denmark (56). A type of utero and lactational exposure of children to relatively low dioxin doses have been found to have permanently impact on reducing sperm quality (57). Gaspari et al. supported that the increased risk of cryptorchidism and hypospadias were related to parental occupational exposure to pesticides (58). Another study reported that consumption of fruits and vegetables with high levels of pesticide residues was associated with a lower total sperm count and a lower percentage of morphologically normal sperm among men presenting to a fertility clinic (59). A newer Chinese study recruiting 1,040 men from the Reproductive Center suggested that environmental exposure to di-n-butyl phthalate (DBP) and di 2-ethyl hexyl phthalate (DEHP) may contribute to a decline in semen quality (60). Another experiment conducted by Huang et al. show that in utero exposure to phthalates has generally threatened the health of newborns (61). There is evidence that PAEs and possibly other EEDs disrupt early male reproductive development, but additional studies are needed.
Female reproduction and development
EEDs may effect on female reproductive system when regarding to the diseases including irregularities in menstruation cycle, precocious puberty, polycystic ovary syndrome and primary ovarian failure (62).
Uterine fibroids (leiomyomas) occurring in 25% to 50% of all women are the most common tumors in female ones (63). Obesity, unopposed estrogen signaling, age during premenopausal years and at menarche have been shown to increase the risks of the development of uterine fibroids (64). Furthermore, there are studies showing that exposure to EEDs can also increase the incidence of fibroids in humans. Shen et al. recruited the subjects including 600 patients with uterine leiomyoma and 600 patients with non-uterine leiomyoma or healthy volunteers, they found that exposure to plastic products, cosmetics, and other chemicals probably containing EEDs may be the risk factor for uterine leiomyoma (65).
Some other side effects of EEDs in female like increased growth of endometrium and higher risk of breast cancer can also occur because of the EEDs (66). In addition, utero exposure of women to diethylstilbestrol (DES) is associated with a high lifetime risk of a broad spectrum of adverse health outcomes (infertility, preterm delivery, ectopic pregnancy, preeclampsia and stillbirth) (67). Altered cyclicity and body burdens have been noted in individuals exposed to OCPs (68). Indeed, cycle irregularities caused by epigenetic changes that may be transmitted to the next generation have been reported in female whose mothers were exposed in utero to DES (69). Meeker et al. found that PAEs exposure is prevalent among third-trimester urinary of pregnant women in Mexico and that some phthalates may be associated with preterm birth (70). Based on a pilot nested case control study, Cantonwine D also supported that low level BPA exposure may impact placental tissue and that urinary concentrations of BPA during the last trimester of pregnancy may relate to risk of delivering prematurely (71). In a study conducted in Taiwan on 33 girls of the general population, PCBs may result in lower estradiol concentrations in 8-year old children and impaired reproductive development in girls (12).
Thyroid function
Normal human metabolic control, for example, brain development, control of metabolism and many other important aspects of normal adult physiology are regulated by thyroid hormones. Disruption in thyroid hormones may results in brain damage or disruptions in metabolism, development and adult physiology if there are changes in the function of the thyroid gland (72).
OCPs as potential EEDs have been reported to influence the level of thyroid hormones, Blanco-Muñoz et al. have observed positive associations between the serum levels of p,p'-DDE and those of total T3 and total T4 (73). Data showed that exposure of children to OCPs produced a significant increase in serum total T3 concentrations (74). A positive association had been observed by Freire between exposure methoxychlor in males and presence of TPOAb, but not in females. He also found that TSH levels were associated with higher beta-HCH in men (75). These results suggest that OCPs can affect the thyroid system through gender-specific mechanisms that may differ among compounds. Chevrier et al. suggested that dioxin exposure, particularly exposure before menarche, may have enduring impacts on women’s total thyroxine levels (76). During the first trimester of pregnancy, exposure to burden of OH-PCBs at environmental levels can affect neonatal thyroid hormone status (77). There was also a tendency toward lower total T4 and higher free T3 with increasing PBDEs exposure. Jacobson et al. found that exposure to PBDEs could breakdown thyroid hormone function, with impacts in the direction of hypothyroidism (78). However, further detailed investigations and health monitoring should be warranted for humans.
Effect on nervous system
Nervous system is one of the most important body systems, it is needed to keep all the body parts synchronized, whereas it can be affected by endocrine-associated chemicals. Neural, behavioral and bipolar disorders have been observed in adults, infants and children due to exposure to EEDs that target different hormonal pathways (79-81). EEDs directly act on many neuro-steroids effecting brain regions causing schizophrenia and bipolar disorders, these steroids evoke psychiatric disorders that can be caused by many pathways like physical development, brain anatomy, cellular anatomy, neurotransmitters and receptors, hormone function, sexual development, immunology, social behaviors or physiological responses (82). There are researchers agreeing on the hypothesis that hypothalamic-pituitary-adrenal (HPA) axis activity may be relevant to the development and expression of psychotic disorders (83,84). Elevated glucocorticoid levels resulted from different causes damage hippocampal nerve and give rise to schizophrenia and anxiety disorders exerting change in HPA axis.
There are some studies providing examples about the exposure of EEDs and their effects on behavior, sensory function and other psychiatric and neurological development as well. Lesiak et al. identify non-dioxin-like polychlorinated biphenyls (NDL PCBs) as potential environmental risk factors for neurodevelopmental disorders (85). Caspersen et al. indicated that maternal dietary exposure to PCB-153 or dl-compounds during pregnancy was significantly associated with poorer expressive language skills in preschool girls (86). In addition, BPA has shown a close association with schizophrenia and other neuro-toxicological pathology (82). The association of perinatal dioxin exposure and autistic traits in 3-year-old children living in a contaminated area in Vietnam were reported, in the study, the high total dioxin-exposed group had significantly more poor neuro-developmental scores than the mild-exposed group in boys, demonstrating a specific impact of perinatal exposure to dioxin on autistic traits in children (81). There was also a case-control study aiming evaluation of level of essential trace elements and heavy metals in the hair samples of children with autistic spectrum disorder (ASD), the result showed high contamination of heavy metals such as lead, mercury and cadmium in ASD children compared to healthy ones, suggesting a possible pathophysiological role of heavy metals in the symptoms of ASD (87).
Obesity and diabetes
Endocrine disruption is a major cause for obesity, which further associate with metabolic disorders, such as diabetes, dyslipidemia, cardiac arrest hypertension, hyperinsulinemia and insulin resistance (88). Glucose metabolism includes estrogenic receptors (ERα and ERβ) as the key parameters. Estradiol (E2) and some EEDs (pesticides, dioxin and BPA) are similar in the way attacking these receptors to evoke changes of glucose homeostasis and insulin release (89,90), so any EED targeting these receptors can damage the normal glucose homeostasis, giving a necessary clue for diabetes (91).
Indeed, there is a growing body of literature involving EEDs such as PCBs, BPA, dioxin and pesticides with the incidence of metabolic syndrome, obesity and diabetes (92-94). Epidemiological studies established a link between type 2 diabetes mellitus (T2DM) and low-level environmental exposure to some dioxin and PCBs, which mainly accumulate in adipose tissue (95). The rapid rise in obesity and diabetes in young suggests that the influence of early-life exposure to EEDs may play an important role in the development of abnormal. Verhulst et al. demonstrated that cord blood PCB and DDE concentrations were associated with increased BMI or change during early childhood (from ages 1 to 3 years) (96). A cross-sectional study included 3,390 adults aged 40 yr or older indicated that BPA was positively associated with generalized obesity, abdominal obesity, and insulin resistance (97). Actually, even low doses of BPA have been indicated to damage α-cells of the pancreas which produce glucagon controlling glucose metabolism (98), thus the low doses of BPA can link to type 2 diabetes and obesity by causing hyperinsulinemia (99). Huang et al. thought that exposure to dioxin being recognized as an EED is a risk factor for diabetes mellitus (DM), independent of age and BMI in both men and women (100). A kind of cross-sectional data reported several positive associations between PAE metabolites and obesity in girls (101). Al-Othman et al. confirmed the association between the body burden of the pesticide hexachlorocyclohexane (HCH) and the risk of T2DM in a sample of 280 adult subjects from Saudi Arabia (102).
Growing evidence also suggests that EEDs may affect not only the exposed individual but also the children and subsequent generations. However, EEDs cause a significant challenge to environment and human health. Consequently, due to their wide commercial use and adverse effect to human health the Endocrine Society had published a scientific statement indicating that endocrine disruptors pose a “significant concern for public health” (2). More research is in urgently needed to provide important insights into the etiology of these chronic disorders caused by EEDs, playing an important role in the design of effective prevention strategies.
Acknowledgments
We thank all subjects who participated in this study and the Suzhou Biobank.
Funding: This study was supported by the scientific projects of Jiangsu Preventive Medicine (Y2013008); and Medical Science and Technology Development Foundation, Nanjing Department of Health (YKK14169).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2016.12.09). BZ serves as an Editor-in-Chief of Journal of Public Health and Emergency from Jan 2017 to Dec 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kavlock RJ, Daston GP, DeRosa C, et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect 1996;104:715-40. [Crossref] [PubMed]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 2009;30:293-342. [Crossref] [PubMed]
- Schug TT, Janesick A, Blumberg B, et al. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol 2011;127:204-15. [Crossref] [PubMed]
- Kim SK, Kim KS, Sang HH. Overview on relative importance of house dust ingestion in human exposure to polybrominated diphenyl ethers (PBDEs): International comparison and Korea as a case. Sci Total Environ 2016;571:82-91. [Crossref] [PubMed]
- Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assist environmental medicine physicians. Altern Med Rev 2010;15:101-9. [PubMed]
- Li WL, Huo CY, Liu LY, et al. Multi-year air monitoring of legacy and current-use brominated flame retardants in an urban center in northeastern China. Sci Total Environ 2016;571:633-42. [Crossref] [PubMed]
- de Boer J, Ballesteros-Gomez A, Leslie HA, et al. Flame retardants: Dust - And not food - Might be the risk. Chemosphere 2016;150:461-4. [Crossref] [PubMed]
- Aschebrook-Kilfoy B, DellaValle CT, Purdue M, et al. Polybrominated diphenyl ethers and thyroid cancer risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial cohort. Am J Epidemiol 2015;181:883-8. [Crossref] [PubMed]
- Lee DH, Steffes MW, Sjodin A, et al. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One 2011;6:e15977 [Crossref] [PubMed]
- Esser A, Schettgen T, Gube M, et al. Association between polychlorinated biphenyls and diabetes mellitus in the German HELPcB cohort. Int J Hyg Environ Health 2016;219:557-65. [Crossref] [PubMed]
- Gao Y, Chen L, Wang C, et al. Exposure to polybrominated diphenyl ethers and female reproductive function: A study in the production area of Shandong, China. Sci Total Environ 2016;572:9-15. [Crossref] [PubMed]
- Su PH, Huang PC, Lin CY, et al. The effect of in utero exposure to dioxins and polychlorinated biphenyls on reproductive development in eight year-old children. Environ Int 2012;39:181-7. [Crossref] [PubMed]
- Terrell ML, Rosenblatt KA, Wirth J, et al. Breast cancer among women in Michigan following exposure to brominated flame retardants. Occup Environ Med 2016;73:564-7. [Crossref] [PubMed]
- Chen XP, Li CW. Progress in research on central nervous system development toxicity of organophosphorus pesticide. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2010;28:545-7. [PubMed]
- Meeker JD. Exposure to environmental endocrine disruptors and child development. Arch Pediatr Adolesc Med 2012;166:952-8. [Crossref] [PubMed]
- Makelarski JA, Romitti PA, Rocheleau CM, et al. Maternal periconceptional occupational pesticide exposure and neural tube defects. Birth Defects Res A Clin Mol Teratol 2014;100:877-86. [Crossref] [PubMed]
- Iaglova NV, Iaglov VV. Alteration of thyroid hormone secretion after long-term exposure to low doses of endocrine disruptor DDT. Biomed Khim 2014;60:655-60. [Crossref] [PubMed]
- Al-Othman AA, Abd-Alrahman SH, Al-Daghri NM. DDT and its metabolites are linked to increased risk of type 2 diabetes among Saudi adults: a cross-sectional study. Environ Sci Pollut Res Int 2015;22:379-86. [Crossref] [PubMed]
- Mangochi P. Endocrine distrupting chemicals and human health: the plausibility of research results on DDT and reproductive health. Malawi Med J 2010;22:42-5. [Crossref] [PubMed]
- Soto AM, Sonnenschein C. Endocrine disruptors: DDT, endocrine disruption and breast cancer. Nat Rev Endocrinol 2015;11:507-8. [PubMed]
- Calafat AM, Weuve J, Ye X, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 2009;117:639-44. [Crossref] [PubMed]
- vom Saal FS, Akingbemi BT, Belcher SM, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol 2007;24:131-8. [Crossref] [PubMed]
- Laing LV, Viana J, Dempster EL, et al. Bisphenol A causes reproductive toxicity, decreases dnmt1 transcription, and reduces global DNA methylation in breeding zebrafish (Danio rerio). Epigenetics 2016;11:526-38. [Crossref] [PubMed]
- Nagao T, Kawachi K, Kagawa N, et al. Neurobehavioral evaluation of mouse newborns exposed prenatally to low-dose bisphenol A. J Toxicol Sci 2014;39:231-5. [Crossref] [PubMed]
- Mínguez-Alarcón L, Hauser R, Gaskins AJ. Effects of bisphenol A on male and couple reproductive health: a review. Fertil Steril 2016;106:864-70. [Crossref] [PubMed]
- Ahmed RG. Maternal bisphenol A alters fetal endocrine system: Thyroid adipokine dysfunction. Food Chem Toxicol 2016;95:168-74. [Crossref] [PubMed]
- Chrysant SG. Association of Exposure to Bisphenol A and Incidence of Cardiovascular Disease and Hypertension. J Clin Hypertens (Greenwich) 2015;17:737-9. [Crossref] [PubMed]
- Ahmadkhaniha R, Mansouri M, Yunesian M, et al. Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J Environ Health Sci Eng 2014;12:64. [Crossref] [PubMed]
- Bao J, Wang M, Ning X, et al. Phthalate concentrations in personal care products and the cumulative exposure to female adults and infants in Shanghai. J Toxicol Environ Health A 2015;78:325-41. [Crossref] [PubMed]
- Hannon PR, Niermann S, Flaws JA. Acute Exposure to Di(2-Ethylhexyl) Phthalate in Adulthood Causes Adverse Reproductive Outcomes Later in Life and Accelerates Reproductive Aging in Female Mice. Toxicol Sci 2016;150:97-108. [Crossref] [PubMed]
- Hao C, Cheng X, Xia H, et al. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep 2012;32:619-29. [Crossref] [PubMed]
- Johns LE, Ferguson KK, Soldin OP, et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol 2015;13:4. [Crossref] [PubMed]
- Stahlhut RW, van Wijngaarden E, Dye TD, et al. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect 2007;115:876-82. [Crossref] [PubMed]
- Wang C, Yang L, Wang S, et al. The classic EDCs, phthalate esters and organochlorines, in relation to abnormal sperm quality: a systematic review with meta-analysis. Sci Rep 2016;6:19982. [Crossref] [PubMed]
- Kim SH, Park MJ. Phthalate exposure and childhood obesity. Ann Pediatr Endocrinol Metab 2014;19:69-75. [Crossref] [PubMed]
- Sun Q, Cornelis MC, Townsend MK, et al. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses' Health Study (NHS) and NHSII cohorts. Environ Health Perspect 2014;122:616-23. [PubMed]
- Cooke GM. Biomonitoring of human fetal exposure to environmental chemicals in early pregnancy. J Toxicol Environ Health B Crit Rev 2014;17:205-24. [Crossref] [PubMed]
- Song Q, Li J. A review on human health consequences of metals exposure to e-waste in China. Environ Pollut 2015;196:450-61. [Crossref] [PubMed]
- Viet SM, Rogers J, Marker D, et al. Lead, allergen, and pesticide levels in licensed child care centers in the United States. J Environ Health 2013;76:8-14. [PubMed]
- Doumouchtsis KK, Doumouchtsis SK, Doumouchtsis EK, et al. The effect of lead intoxication on endocrine functions. J Endocrinol Invest 2009;32:175-83. [Crossref] [PubMed]
- Kortenkamp A. Are cadmium and other heavy metal compounds acting as endocrine disrupters? Met Ions Life Sci 2011;8:305-17. [PubMed]
- Minoia C, Ronchi A, Pigatto P, et al. Effects of mercury on the endocrine system. Crit Rev Toxicol 2009;39:538-author reply 539. [Crossref] [PubMed]
- Guo Z, Guo H, Xia Y. Effects on endocrine system of female rats exposed to chronic arsenic. Wei Sheng Yan Jiu 2011;40:178-9. [PubMed]
- Feng Y, Zhang P, Zhang Z, et al. Endocrine Disrupting Effects of Triclosan on the Placenta in Pregnant Rats. PLoS One 2016;11:e0154758 [Crossref] [PubMed]
- Boberg J, Taxvig C, Christiansen S, et al. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol 2010;30:301-12. [Crossref] [PubMed]
- Park JW, Rinchard J, Liu F, et al. The thyroid endocrine disruptor perchlorate affects reproduction, growth, and survival of mosquitofish. Ecotoxicol Environ Saf 2006;63:343-52. [Crossref] [PubMed]
- Du G, Hu J, Huang H, et al. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ Toxicol Chem 2013;32:353-60. [Crossref] [PubMed]
- Main KM, Skakkebaek NE, Virtanen HE, et al. Genital anomalies in boys and the environment. Best Pract Res Clin Endocrinol Metab 2010;24:279-89. [Crossref] [PubMed]
- Fechner P, Damdimopoulou P, Gauglitz G. Biosensors paving the way to understanding the interaction between cadmium and the estrogen receptor alpha. PLoS One 2011;6:e23048 [Crossref] [PubMed]
- Bonde JP, Giwercman A. Environmental xenobiotics and male reproductive health. Asian J Androl 2014;16:3-4. [Crossref] [PubMed]
- Zhu W, Zhang H, Tong C, et al. Environmental Exposure to Triclosan and Semen Quality. Int J Environ Res Public Health 2016;13:224. [Crossref] [PubMed]
- Radwan M, Jurewicz J, Wielgomas B, et al. Semen quality and the level of reproductive hormones after environmental exposure to pyrethroids. J Occup Environ Med 2014;56:1113-9. [Crossref] [PubMed]
- Pant N, Shukla M, Upadhyay AD, et al. Association between environmental exposure to p, p'-DDE and lindane and semen quality. Environ Sci Pollut Res Int 2014;21:11009-16. [Crossref] [PubMed]
- Toppari J, Virtanen HE, Main KM, et al. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res A Clin Mol Teratol 2010;88:910-9. [Crossref] [PubMed]
- Skakkebaek NE, Rajpert-De ME, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001;16:972-8. [Crossref] [PubMed]
- Main KM, Kiviranta H, Virtanen HE, et al. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect 2007;115:1519-26. [PubMed]
- Mocarelli P, Gerthoux PM, Needham LL, et al. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect 2011;119:713-8. [Crossref] [PubMed]
- Gaspari L, Paris F, Jandel C, et al. Prenatal environmental risk factors for genital malformations in a population of 1442 French male newborns: a nested case-control study. Hum Reprod 2011;26:3155-62. [Crossref] [PubMed]
- Chiu YH, Afeiche MC, Gaskins AJ, et al. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum Reprod 2015;30:1342-51. [Crossref] [PubMed]
- Wang YX, You L, Zeng Q, et al. Phthalate exposure and human semen quality: Results from an infertility clinic in China. Environ Res 2015;142:1-9. [Crossref] [PubMed]
- Huang PC, Kuo PL, Chou YY, et al. Association between prenatal exposure to phthalates and the health of newborns. Environ Int 2009;35:14-20. [Crossref] [PubMed]
- Costa EM, Spritzer PM, Hohl A, et al. Effects of endocrine disruptors in the development of the female reproductive tract. Arq Bras Endocrinol Metabol 2014;58:153-61. [Crossref] [PubMed]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science 2005;308:1589-92. [Crossref] [PubMed]
- Kjerulff KH, Langenberg P, Seidman JD, et al. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med 1996;41:483-90. [PubMed]
- Shen Y, Xu Q, Xu J, et al. Environmental exposure and risk of uterine leiomyoma: an epidemiologic survey. Eur Rev Med Pharmacol Sci 2013;17:3249-56. [PubMed]
- Tournaire M, Devouche E, Espie M, et al. Cancer Risk in Women Exposed to Diethylstilbestrol in Utero. Therapie 2015;70:433-41. [Crossref] [PubMed]
- Hoover RN, Hyer M, Pfeiffer RM, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med 2011;365:1304-14. [Crossref] [PubMed]
- Kanazawa A, Miyasita C, Okada E, et al. Blood persistent organochlorine pesticides in pregnant women in relation to physical and environmental variables in The Hokkaido Study on Environment and Children's Health. Sci Total Environ 2012;426:73-82. [Crossref] [PubMed]
- Titus-Ernstoff L, Troisi R, Hatch EE, et al. Offspring of women exposed in utero to diethylstilbestrol (DES): a preliminary report of benign and malignant pathology in the third generation. Epidemiology 2008;19:251-7. [Crossref] [PubMed]
- Meeker JD, Hu H, Cantonwine DE, et al. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect 2009;117:1587-92. [Crossref] [PubMed]
- Cantonwine D, Meeker JD, Hu H, et al. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ Health 2010;9:62. [Crossref] [PubMed]
- Dezonne RS, Lima FR, Trentin AG, Gomes FC. Thyroid hormone and astroglia: endocrine control of the neural environment. J Neuroendocrinol 2015;27:435-45. [Crossref] [PubMed]
- Blanco-Muñoz J, Lacasaña M, López-Flores I, et al. Association between organochlorine pesticide exposure and thyroid hormones in floriculture workers. Environ Res 2016;150:357-63. [Crossref] [PubMed]
- Freire C, Koifman RJ, Sarcinelli P, et al. Long term exposure to organochlorine pesticides and thyroid function in children from Cidade dos Meninos, Rio de Janeiro, Brazil. Environ Res 2012;117:68-74. [Crossref] [PubMed]
- Freire C, Koifman RJ, Sarcinelli PN, et al. Long-term exposure to organochlorine pesticides and thyroid status in adults in a heavily contaminated area in Brazil. Environ Res 2013;127:7-15. [Crossref] [PubMed]
- Chevrier J, Warner M, Gunier RB, et al. Serum dioxin concentrations and thyroid hormone levels in the Seveso Women's Health Study. Am J Epidemiol 2014;180:490-8. [Crossref] [PubMed]
- Hisada A, Shimodaira K, Okai T, et al. Associations between levels of hydroxylated PCBs and PCBs in serum of pregnant women and blood thyroid hormone levels and body size of neonates. Int J Hyg Environ Health 2014;217:546-53. [Crossref] [PubMed]
- Jacobson MH, Barr DB, Marcus M, et al. Serum polybrominated diphenyl ether concentrations and thyroid function in young children. Environ Res 2016;149:222-30. [Crossref] [PubMed]
- John U, Meyer C, Rumpf HJ, et al. Psychiatric comorbidity including nicotine dependence among individuals with eating disorder criteria in an adult general population sample. Psychiatry Res 2006;141:71-9. [Crossref] [PubMed]
- Masuo Y, Ishido M. Neurotoxicity of endocrine disruptors: possible involvement in brain development and neurodegeneration. J Toxicol Environ Health B Crit Rev 2011;14:346-69. [Crossref] [PubMed]
- Nishijo M, Pham TT, Nguyen AT, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin in breast milk increases autistic traits of 3-year-old children in Vietnam. Mol Psychiatry 2014;19:1220-6. [Crossref] [PubMed]
- Brown JS Jr. Effects of bisphenol-A and other endocrine disruptors compared with abnormalities of schizophrenia: an endocrine-disruption theory of schizophrenia. Schizophr Bull 2009;35:256-78. [Crossref] [PubMed]
- Corcoran CM, Smith C, McLaughlin D, et al. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr Res 2012;135:170-4. [Crossref] [PubMed]
- Cotter D, Pariante CM. Stress and the progression of the developmental hypothesis of schizophrenia. Br J Psychiatry 2002;181:363-5. [Crossref] [PubMed]
- Lesiak A, Zhu M, Chen H, et al. The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. J Neurosci 2014;34:717-25. [Crossref] [PubMed]
- Caspersen IH, Aase H, Biele G, et al. The influence of maternal dietary exposure to dioxins and PCBs during pregnancy on ADHD symptoms and cognitive functions in Norwegian preschool children. Environ Int 2016;94:649-60. [Crossref] [PubMed]
- Tabatadze T, Zhorzholiani L, Kherkheulidze M, et al. Hair heavy metal and essential trace element concentration in children with autism spectrum disorder. Georgian Med News 2015;77-82. [PubMed]
- Newbold RR, Padilla-Banks E, Jefferson WN, et al. Effects of endocrine disruptors on obesity. Int J Androl 2008;31:201-8. [Crossref] [PubMed]
- Le May C, Chu K, Hu M, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A 2006;103:9232-7. [Crossref] [PubMed]
- Nadal A, Alonso-Magdalena P, Soriano S, et al. The pancreatic beta-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol 2009;304:63-8. [Crossref] [PubMed]
- Ropero AB, Alonso-Magdalena P, Quesada I, et al. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids 2008;73:874-9. [Crossref] [PubMed]
- Ghosh S, Murinova L, Trnovec T, et al. Biomarkers linking PCB exposure and obesity. Curr Pharm Biotechnol 2014;15:1058-68. [Crossref] [PubMed]
- Frugé AD, Cases MG, Schildkraut JM, et al. Associations between Obesity, Body Fat Distribution, Weight Loss and Weight Cycling on Serum Pesticide Concentrations. J Food Nutr Disord 2016;5. [PubMed]
- Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso women's health study. Environ Health Perspect 2013;121:906-11. [Crossref] [PubMed]
- Uemura H. Associations of exposure to dioxins and polychlorinated biphenyls with diabetes: based on epidemiological findings. Nihon Eiseigaku Zasshi 2012;67:363-74. [Crossref] [PubMed]
- Verhulst SL, Nelen V, Hond ED, et al. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ Health Perspect 2009;117:122-6. [Crossref] [PubMed]
- Wang T, Li M, Chen B, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 2012;97:E223-7. [Crossref] [PubMed]
- Alonso-Magdalena P, Laribi O, Ropero AB, et al. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect 2005;113:969-77. [Crossref] [PubMed]
- Alonso-Magdalena P, Vieira E, Soriano S, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect 2010;118:1243-50. [Crossref] [PubMed]
- Huang CY, Wu CL, Yang YC, et al. Association between Dioxin and Diabetes Mellitus in an Endemic Area of Exposure in Taiwan: A Population-Based Study. Medicine (Baltimore) 2015;94:e1730 [Crossref] [PubMed]
- Hatch EE, Nelson JW, Stahlhut RW, et al. Association of endocrine disruptors and obesity: perspectives from epidemiological studies. Int J Androl 2010;33:324-32. [Crossref] [PubMed]
- Al-Othman A, Yakout S, Abd-Alrahman SH, et al. Strong associations between the pesticide hexachlorocyclohexane and type 2 diabetes in Saudi adults. Int J Environ Res Public Health 2014;11:8984-95. [Crossref] [PubMed]
Cite this article as: Li X, Gao Y, Wang J, Ji G, Lu Y, Yang D, Shen H, Dong Q, Pan L, Xiao H, Zhu B. Exposure to environmental endocrine disruptors and human health. J Public Health Emerg 2017;1:8.