
Theoretical clearance of diesel exhaust particles in the lungs of children with different ages
Introduction
As already outlined in numerous previous publications (1-10), diesel exhaust particles (DEP) are generated by combustion processes taking place in diesel engines. They either occur as single particles, which have been formed by complex nucleation processes, or infiltrate the ambient atmosphere as agglomerates containing a multitude of variably sized and shaped components. In general, DEP range in size from 100 to 250 nm and thus belong to the large category of sub-micron particles that are characterized by enhanced inhalability (1-3). According to modern medical research these specific particles can be related to a high number of lung diseases of different severity, among which chronic bronchitis and asthma occur with highest frequencies (7-13).
Uptake of DEP by inhalation has several consequences for the human body. Depending on particle size and intensity of breathing, between 30 and 50% of the particulate mass are deposited in single compartments of the respiratory tract. Thereby, highest deposition rates can be registered for the bronchial and bronchiolar airways, whilst significantly lower amounts of particles are accumulated in tubular structures of the extrathoracic region and the alveoli (11-16). Immediately after their collision with the airway walls or alveolar epithelia, the particles are confronted with the innate defence system, which consists of several site-specific clearance mechanisms with different velocities and efficiencies with regard to the removal the particulate mass (17-25). In the bronchial and bronchiolar airways clearance is mainly dominated by the so-called mucociliary escalator, which represents a mucous blanket lining the epithelial walls and being transported towards the trachea due to the activity of ciliary cells. Almost all particles captured on this layer are removed from the respiratory tract within 24 hours after the inhalation and deposition event (20-25). Those particles passing the mucous blanket and reaching the epithelial surface are seized by slow bronchial clearance mechanisms that among other include the re-transfer of particulate mass on the escalator, removal of deposited substances by airway macrophages and transepithelial particle movement towards the lymphatic and cardiovascular system (26-30). Particles deposited in the alveolar structures mainly undergo a macrophage-related clearance process. Alternatively, they can be also subjected to transepithelial removal or short-range transport events on the surfactant layer, whereby in the latter case they can be again channelled into the airway system (31-33).
According to several theoretical studies carried out in the past decades, total velocity of particle clearance in the human respiratory tract exhibits a significant dependence on subject’s age. Tracheal mucus velocity is subject to a continuous increase from infancy to adulthood and to a slight decline from adulthood to senectitude (34,35). As a consequence of this phenomenon, efficiency of the mucociliary escalator is enhanced with proceeding age in the early period of life, but decreased with proceeding age in the late period of life. It is further hypothesized that slow bronchial and alveolar clearance mechanisms are only marginally influenced by subject’s age (34,35).
In the present contribution lung clearance of DEP ranging in size from 100 to 250 nm (density: 2.2 g·cm−3) was theoretically investigated for children belonging to two age groups (5, 10 years). Preceding deposition scenarios of inhaled particles were simulated for two breathing scenarios, i.e., sitting inhalation and light-exercise inhalation (36). With the help of the clearance model described in the next section specific particle retention values (24-h retention, 10-d retention) as well as total clearance times were computed. In addition, particle-related clearance curves reflecting different phases of mass removal were generated. Respective discrepancies of the clearance behaviour between the age groups were subjected to an intense debate.
Methods
Stochastic model of bronchial and alveolar clearance
Since the stochastic clearance model has been outlined in detail in previous publications (20-30), only the most salient features of this well validated approach will be brought up in this contribution. Basically, the approach distinguishes between tracheobronchial clearance on the one hand and alveolar clearance on the other. Tracheobronchial clearance can be subdivided into fast mucociliary clearance with half-times of several hours and slow clearance processes with half-times ranging from 5 to 20 days (20-25,36). Removal of deposited particles on the cilia-driven mucus blanket lining the single airway walls is approximated by the definition of site-specific mucus velocities. These velocities are calculated under the assumption of a steady-state mucus flow taking place from the terminal bronchioles towards the trachea. It is also supposed that the mucus blanket continuously declines in thickness from central to peripheral airways due to a permanent reduction of the secretory activity with rising airway generation. Finally, the mucus layer is characterized by specific discontinuities resulting from coughing processes and inhomogeneous secretion, so that inhaled particles get the chance of directly hitting the epithelial surface. Site-specific mucus velocities are derived from mucus volume balancing between parent and daughter generations in a given airway bifurcation, whereby locally occurring mucus volumes among other depend on the geometric properties (diameter, length) of single airway tubes. In the end, velocities of the mucus blanket exhibit an exponential decrease from the trachea to the terminal bronchioles situated in the outermost parts of the lungs.
Slow bronchial clearance was modelled by defining a respective clearance fraction (fs) on the basis of a linear regression function, which describes the amount of this parameter in dependency on the geometric diameter of the deposited particles (20-25). Since the slow clearance process taking place in the bronchial and bronchiolar airway tubes can be subdivided into several mechanisms with different velocities of particle removal (e.g., re-capture of particles on the mucus blanket, particle uptake by airway macrophages, transcytosis), half-time of this procedure was stochastically varied between 5 and 20 days, resulting in total clearance times between 25 and 100 days.
With regard to alveolar clearance a similar strategy as described for slow bronchial clearance was pursued. Therefore, faster particle removal induced by alveolar macrophages was separated from much slower processes including particle transport on the surfactant as well as transcytosis with related storage of particulate mass in the interstitium (31-36). Whilst half-time of macrophage clearance was assumed to vary between 5 and 20 days, half-time of all slower mechanisms was constituted with 20 to 100 days. Definition of respective particle fractions belonging either to fast or to slow alveolar clearance processes was again conducted by means of particle size-related regression functions (31-36).
Modelling lung morphometry of 5- and 10-year-old children
In general, children are characterized by significantly smaller lungs than adults. As demonstrated in previous studies (34-36), lung morphometry of 5-year-old children corresponds to 51.7% of the respective morphometric values measured in adults. Size of the respiratory tract determined in 10-year-old children, on the other hand, corresponds to 66.5% of the size determined in adult probands. This remarkable age-related reduction in size has considerable consequences on both deposition and clearance of inhaled particles. Under the assumption of a tracheal mucus velocity of 15 mm·min−1 in adults (34,35), velocity of the mucus blanket in the trachea adopts values of 5 mm·min−1 in 5-year-old children and 8 mm·min−1 in 10-year-old children due to the age-specific morphometries noted above. Mucus velocities of the following airway generations were computed on the basis of the tracheal velocities stated above and the age-related lung morphometries. For the sake of simplicity, physical activity of the probands (sitting or light-exercise activity) was not evaluated as decisive factor exerting its influence on bronchial and bronchiolar mucus transport.
Results
Retention values and total clearance times
In order to obtain a comprehensive picture of DEP clearance in children of different age, 24-h and 10-d retention values were modelled for particles with different size and for different breathing conditions resulting in different particle deposition patterns (Figures 1,2). In 5-year-old children, who were exposed to DEP under sitting breathing conditions, 24-h retention adopts values between 93.7% and 95.7%, whereas in 10-day-old children, who were confronted with DEP under the same conditions, the respective parameter ranges from 90.7% to 93.4%. Retention values measured after 10 days vary between 68.3% and 77.7% in 5-year old probands, but between 56.5% and 66.8% in 10-year-old probands. Generally, a slight decrease of particle retention with rising DEP size can be observed (Figure 1). If the children were exposed to DEP under light-exercise breathing conditions, 24-h retention adopts values between 95.4% and 96.0% (5 years) and between 93.2% and 94.0% (10 years), whilst 10-d retention ranges from 76.0% to 78.9% (5 years) and from 66.0% to 69.7% (10 years).

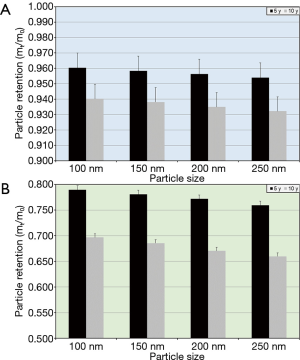
Total clearance times computed by the model range from 131 to 223 d in 5-year-old children and from 91 to 125 d in 10-year-old children. Here, size of deposited DEP has an effect insofar as respective periods of intrapulmonary particle residence are continuously shortened with rising particle diameter. Any switch of the breathing conditions, under which uptake of DEP took place, has considerable effects on total clearance times. Concretely speaking, respective values modelled for 5-year-old children range from 176 to 259 d, whereas values computed for 10-year-old probands vary between 121 and 138 d (Figure 2).
Short-term and long-term course of DEP clearance in children
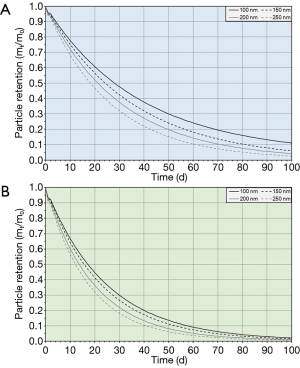
Clearance curves calculated for different particle sizes and probands adopting different ages are summarized in Figures 3 and 4. Clearance of DEP following particle exposure under sitting breathing conditions can be subdivided into three distinct phases: A fast phase taking place within the first 24 h after DEP inhalation is superseded by an intermediate phase with a total duration of about 25 d and a very slow phase with a total duration of several hundred days. This clearance behaviour, however, can be observed in all probands, whereby any dependence of the clearance course on the size of the deposited particles and the physical conditions during breathing is mainly reflected in the early phases of this innate defence system.

Discussion
Based upon the results presented in the previous section, some fundamental statements on the clearance of DEP in children’s lungs can be made: (I) particle retention and total clearance times exhibit a negative correlation with the size of the inhaled particulate substances. This means that larger DEP are faster evacuated from the single lung compartments than smaller DEP. (II) Particles deposited in 5-year-old children are subjected to an innate defence process, which works remarkably slower than that in 10-year-old children. Therefore, clearance efficiency can be clearly related to the age of the probands included in the study. (III) If conditions of particle uptake (e.g., by switching from sitting to light-exercise breathing) are changed, this also results in consequences for the clearance of particulate mass deposited in the airways and alveoli. In the case of DEP any intensification of particle inhalation leads to deposition scenarios, which may cause a valuable prolongation of the total clearance time.
Concerning the relationship between clearance velocity and particle size, it has to be stated that DEP of different size generate different deposition patterns in the respiratory tracts of probands with different ages. As already outlined in previous studies (37-50), within a particle size range of 0.1 to 1.0 µm extrathoracic and intrapulmonary deposition are subject to several changes due to a continuous unbalancement of respective deposition mechanisms. Whilst smaller particles are mainly seized by diffusion processes, larger particles are increasingly seized by mass-related processes (48-50). Both types of deposition mechanisms are associated with a preferential accumulation of inhaled particulate matter in the upper and central airways. As already mentioned in the preceding chapters, slow bronchial clearance fraction has to be conceived as a function of particle size, with smaller objects being seized more easily by respective mechanisms than larger ones (20-25). Summing up all these considerations, larger DEP are finally marked by higher clearance efficiencies that are expressed by lower retention values and shortened total clearance times.
The difference of particle clearance between 5- and 10-year-old probands can be mainly ascribed to two essential circumstances: First, younger children exhibit a breathing behaviour, which is significantly different from that of older children and is mainly expressed by shorter inhalation and exhalation periods as well as the complete waiver of a breath-hold (34-36). This phenomenon causes the development of very age-specific particle deposition patterns. Second, the lungs of older children are remarkably larger in size than those in younger probands, which has an effect on the amount of produced mucus and the ciliary transport of the mucus blanket (20-30,33,34). Due to shallower breathing of young children main particulate mass is deposited in the upper and central airways, from where it can be removed by bronchial clearance processes. Higher amounts of mucus, on the other hand, can be related to increased mucus velocities in order to guarantee the maintenance of the whole clearance system (34-36). After assessment of physiological and morphometric effects, clearance of older children is significantly accelerated by this enhanced activity of mucus secretion.
The relationship between clearance of DEP and breathing intensity during particle exposure seems to be incomprehensible at first glance, but can be also related to the deposition patterns generated under the particular conditions of inhalation. Since sub-micron particles are mainly seized by diffusion processes, residence times of particulate objects in single airway generations are decisive for the probability of deposition (36,44-50). Slow inhalation of DEP commonly results in prolonged residence times of these particles in the uppermost airways of the respiratory system, so that they can hit the airway walls more likely. Any increase of the inhalation velocity and volume implicates a drastic shortening of particle residence in the upper airway structures and the preferential transfer of particulate mass to the peripheral compartments (44-50). The consequences of these phenomena on the clearance process have been already discussed in the preceding paragraphs.
Summing up all results presented in this contribution it can be concluded that DEP may bear a hazardous effect in children’s lungs albeit they are cleared from the diverse structures of the respiratory tract in valuable amount. Especially those particles, which are accumulated in the most peripheral airways (i.e., terminal and respiratory bronchioles) as well as in the alveoli, may be subjected to processes of long-term storage in specific epithelial cells. This phenomenon, however, may be responsible for the generation of diverse lung diseases.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2020.02.02). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Groblicki PJ. Particle Size Variation in Diesel Car Exhaust. SAE Technical Paper Series no. 790421. Warrendale: Society of Automotive Engineers, 1979.
- Ullman TL. Investigation of the Effects of Fuel Composition on Heavy Duty Diesel Engine Emissions. SAE Technical Paper No. 892072. Society of Automotive Engineers, 1989.
- McClellan RO. Toxicological effects of emissions from diesel engines. Dev Toxicol Environ Sci 1986;13:3-8. [PubMed]
- Williams RL. Diesel particulate emissions: Composition, concentration, and control. Dev Toxicol Environ Sci 1982;10:15-32. [PubMed]
- Baumgard KJ, Johnson JH. The effect of Fuel and Engine Design on Diesel Exhaust Particle Size Distributions. SAE Technical Paper Series no. 960131. Warrendale: Society of Automotive Engineers, 1996.
- IARC. Diesel and gasoline engine exhausts. In Diesel and Gasoline Engine Exhausts and Some Nitroarenes. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, vol. 46. Lyon: International Agency for Research on Cancer, 1989.
- NTP. Report on Carcinogens Background Document for Diesel Exhaust Particulates. Research Triangle Park: National Toxicology Program, 2000.
- Sturm R. Deposition of diesel exhaust particles in the human lungs: theoretical simulations and experimental data. J Public Health Emerg 2017;1:70. [Crossref]
- Sturm R. Theoretical deposition of random walk-generated nanoaggregates in the lungs of healthy males and females. J Publ Health Emerg 2018;2:4. [Crossref]
- Sturm R. Theoretical deposition of diesel exhaust particles in the respiratory tract of children. J Public Health Emerg 2019;3:12. [Crossref]
- Garshick E, Laden F, Hart JE, et al. Lung cancer and vehicle exhaust in trucking industry workers. Environ Health Perspect 2008;116:1327-32. [Crossref] [PubMed]
- Neumeyer-Gromen A, Razum O, Kersten N, et al. Diesel motor emissions and lung cancer mortality—results of the second follow-up of a cohort study in potash miners. Int J Cancer 2009;124:1900-6. [Crossref] [PubMed]
- Penconek A, Arkadiusz M. Deposition of diesel exhaust particles from various fuels in a cast of human respiratory system under two breathing patterns. J Aerosol Sci 2013;63:48-59. [Crossref]
- Sturm R. Theoretical deposition of carcinogenic particle aggregates in the upper respiratory tract. Ann Transl Med 2013;1:25. [PubMed]
- Sturm R. Inhaled nanoparticles. Phys Today 2016;69:70-1. [Crossref]
- Sturm R. Theoretical deposition of variably sized platelets in the respiratory tract of healthy adults. AME Med J 2018;3:5. [Crossref]
- Sturm R, Hofmann W. A theoretical approach to the deposition and clearance of fibers with variable size in the human respiratory tract. J Hazard Mater 2009;170:210-8. [Crossref] [PubMed]
- Sturm R. Theoretical simulation of diesel exhaust particle clearance from the human respiratory tract. J Public Health Emerg 2017;1:74. [Crossref]
- Sturm R. A computer model for the clearance of insoluble particles from the tracheobronchial tree of the human lung. Comput Biol Med 2007;37:680-90. [Crossref] [PubMed]
- Hofmann W, Sturm R. Stochastic model of particle clearance in human bronchial airways. J Aerosol Med 2004;17:73-89. [Crossref] [PubMed]
- Sturm R. A three-dimensional model of tracheobronchial particle distribution during mucociliary clearance in the human respiratory tract. Z Med Phys 2013;23:111-9. [Crossref] [PubMed]
- Sturm R, Hofmann W, Scheuch G, et al. Particle clearance in human bronchial airways: Comparison of stochastic model predictions with experimental data. Ann Occup Hyg 2002;46:329-33. [PubMed]
- Sturm R, Hofmann W. Mechanistic interpretation of the slow bronchial clearance phase. Radiat Prot Dosimetry 2003;105:101-4. [Crossref] [PubMed]
- Sturm R, Hofmann W. A multi-compartment model for slow bronchial clearance of insoluble particles—extension of the ICRP human respiratory tract models. Radiat Prot Dosimetry 2006;118:384-94. [Crossref] [PubMed]
- Sturm R, Hofmann W. Stochastic modeling predictions for the clearance of insoluble particles from the tracheobronchial tree of the human lung. Bull Math Biol 2007;69:395-415. [Crossref] [PubMed]
- Sturm R. Modeling the delay of mucous flow at the carinal ridges of the human tracheobronchial tree. Comp Math Biol 2014;3:6.
- Svartengren M, Svartengren K, Europe E, et al. Long-term clearance from small airways in patients with chronic bronchitis: experimental and theoretical data. Exp Lung Res 2004;30:333-53. [Crossref] [PubMed]
- Sturm R. Modeling bronchial clearance in the lungs of healthy subjects and smokers. Comp Math Biol 2017;6:2.
- Sturm R. An advanced mathematical model of slow bronchial clearance in the human respiratory tract. Comp Math Biol 2016;5:2.
- Sturm R. Theoretical models for the simulation of particle deposition and tracheobronchial clearance in lungs of patients with chronic bronchitis. Ann Transl Med 2013;1:3. [PubMed]
- Sturm R. An advanced stochastic model for mucociliary particle clearance in cystic fibrosis lungs. J Thorac Dis 2012;4:48-57. [PubMed]
- Hofmann W, Sturm R, Asgharian B. Stochastic simulation of particle clearance in human bronchial airways. J Aerosol Sci 2001;2S807-8.
- Sturm R. Theoretical models of carcinogenic particle deposition and clearance in children’s lungs. J Thorac Dis 2012;4:368-76. [PubMed]
- Sturm R. Bioaerosols in the lungs of subjects with different ages – Part 2: clearance modeling. Ann Transl Med 2017;5:95. [Crossref] [PubMed]
- Sturm R. Age-dependence and intersubject variability of tracheobronchial particle clearance. Pneumon 2011;4:77-84.
- International Commission on Radiological Protection (ICRP). Human respiratory tract model for radiological protection, Publication 66. Oxford: Pergamon Press, 1994.
- Sturm R. Nanotubes in the respiratory tract - Deposition modeling. Z med Phys 2015;25:135-45. [Crossref] [PubMed]
- Sturm R. A stochastic model of carbon nanotube deposition in the airways and alveoli of the human respiratory tract. Inhal Toxicol 2016;28:49-60. [Crossref] [PubMed]
- Sturm R. Spatial visualization of theoretical nanoparticle deposition in the human respiratory tract. Ann Transl Med 2015;3:326. [PubMed]
- Sturm R. Deposition of ultrafine particles with various shapes in the human alveoli – a model approach. Comp Math Biol 2016;5:4.
- Sturm R. Theoretical deposition of nanotubes in the respir¬atory tract of children and adults. Ann Transl Med 2014;2:6. [PubMed]
- Sturm R. Computer-aided generation and lung deposition modeling of nano-scale particle aggregates. Inhal Toxicol 2017;29:160-8. [Crossref] [PubMed]
- Hofmann W, Sturm R, Winkler-Heil R, et al. Stochastic model of ultrafine particle deposition and clearance in the human respiratory tract. Radiat Prot Dosimetry 2003;105:77-80. [Crossref] [PubMed]
- Sturm R. Theoretical and experimental approaches to the deposition and clearance of ultrafine carcinogens in the human respiratory tract. Thorac Cancer 2011;2:61-8. [Crossref] [PubMed]
- Sturm R. A computer model for the simulation of fiber-cell interaction in the alveolar region of the respiratory tract. Comput Biol Med. 2011;41:565-73. [Crossref] [PubMed]
- Sturm R, Hofmann W. A computer program for the simulation of fiber deposition in the human respiratory tract. Comput Biol Med. 2006;36:1252-67. [Crossref] [PubMed]
- Sturm R. Theoretical approach to the hit probability of lung-cancer-sensitive epithelial cells by mineral fibers with various aspect ratios. Thoracic Cancer 2010;1:116-25. [Crossref] [PubMed]
- Sturm R. Modeling the deposition of bioaerosols with variable size and shape in the human respiratory tract–A review. J Adv Res 2012;3:295-304. [Crossref]
- Sturm R. Inhalation of nanoplatelets- theoretical deposition simulations. Z Med Phys 2017;27:274-84. [Crossref] [PubMed]
- Sturm R. Theoretical models for dynamic shape factors and lung deposition of small particle aggregates originating from combustion processes. Z med Phys 2010;20:226-34. [Crossref] [PubMed]
Cite this article as: Sturm R. Theoretical clearance of diesel exhaust particles in the lungs of children with different ages. J Public Health Emerg 2020;4:3.


