
Particles in the lungs of patients with chronic bronchitis—part 1: deposition modeling
Introduction
In medical respects chronic bronchitis may be regarded as so-called chronic obstructive pulmonary disease (COPD) which is distinguished by the progressive development of airflow limitations due to a permanent reduction of the airway calibers. This process is characterized by only partial reversibility (1-3). In addition to chronic bronchitis COPD also encompasses emphysema which is marked by a permanent enlargement of the air spaces distal to the terminal bronchioles. This phenomenon is accompanied by the successive destruction of the lung parenchyma, the loss of lung elasticity and, as a consequence of that, the closure of small airways (1,4). Since patients suffering from chronic bronchitis produce abnormal amounts of mucous liquid, formation of local mucous plugs can lead to a further exacerbation of the lung disease. The typical clinical picture of COPD patients comprises three pathological conditions: chronic bronchitis, emphysema and mucus plugging. Thereby, the relative extent of bronchitis and emphysema may undergo an intersubject variability (1) (Figure 1).
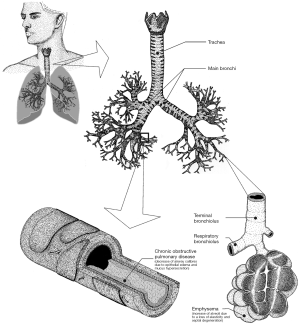
When looked at chronic bronchitis more closely, the disease is defined by the presence of a productive cough with a duration of more than three months in two consecutive years (5). The cough may be understood as a result of the hypersecretion of mucus. Enhanced production of mucous secretion results from the continuous enlargement of mucus glands, whereby this development represents the histological hallmark of the insufficiency (6). The further course of the disease is among other characterized by the occurrence of atrophy, focal squamous metaplasia, abnormalities of epithelial cilia, inflammation and bronchial wall thickening. The last symptom has to be regarded as result of the formation of edema and the accumulation of neutrophilic infiltrates in the submucosa (2). Most considerable modifications have to be attested for the respiratory bronchioles, where mononuclear inflammatory processes are usually accompanied goblet cell metaplasia, smooth muscle hyperplasia and distortion due to fibrosis (3).
Current treatment of chronic bronchitis mainly includes drug therapies, the administration of corticosteroids, non-pharmacological methods and the application of innovative medicals such as mediator antagonists, protease inhibitors or anti-inflammatory drugs (1,7-11). Despite the fact that bronchitis therapy has achieved remarkable progresses during the past decades, only cessation of smoking, representing the primary cause of the disease, leads to a valuable retardation of the developmental stages noted above (12). Medical substances administered by conventional drug therapy include bronchodilators resulting in an enhancement of the forced expiratory volume in 1 s (FEV1) (12,13), antibiotics against bacterial infection and oxygen for the treatment of chronic hypoxemia. Inhaled or ingested corticosteroids do not suppress inflammatory reactions accompanying the disease (14,15), but, on the other hand, they may have a beneficial effect in the treatment of acute exacerbations of COPD (16,17).
As most drugs are targeted to the diseased sites of the lungs in aerosolized form, precise knowledge of the behavior of liquid or solid particles in the human respiratory tract are of immense importance. With regard to this essential question in connection with the therapy of bronchitis patients, theoretical models may provide a considerable contribution which finally results in an optimization of drug delivery through the airway system. In the present contribution a respective mathematical approach dealing with this problem is described in detail. In addition, theoretical prediction of total, regional and local particle deposition are presented for various breathing scenarios. With regard to public health the contribution is of increased value, because (I) chronic bronchitis ranks among the most prominent COPD causing enormous health costs per year; (II) detailed knowledge of particle deposition in chronic bronchitis patients can support the development of more efficient inhalation therapies lowering the costs of medical treatment; (III) with the help of comprehensive and accurate models’ effective strategies against bronchitis and its large-quantity occurrence can be worked out; (IV) the theoretical approach of particle deposition in healthy controls and bronchitis patients can provide significant support concerning the targeted preparation of ethics certificates, which represent the foundation of inhalation therapies and further experimental studies.
Methods
Airway scaling for the simulation of diseased bronchial structures
The modified lung architecture occurring in bronchitis patients was approximated by application of a specific scaling procedure of the tracheobronchial tree. Extent of re-calibration depended on both the functional residual capacity (FRC) and the airway resistance (RAW) of the diseased lungs. For FRC-related airway calibration a uniform scaling factor (SFFRC) was defined for the whole bronchial system (18,19), thereby using the general equation:
In the formula noted above FRCCB represents the FRC of a respiratory system affected by chronic bronchitis, whereas the value of 5,500 mL denotes the total air volume of an average healthy lung belonging to a male adult (19,20). Functional residual capacities of COPD patients were among other measured by Kim & Kang (21) who investigated the deposition of di-2-ethylhexyl sebacate oil particles with a diameter of 1 µm in the lungs of 10 probands exhibiting different degrees of the lung insufficiency.
Total RAW indicted by the RAW value is subject to an increase with the exacerbation grade of chronic bronchitis. Due to this important circumstance this parameter may be used as appropriate tool for a determination of the diameter reduction in the air-conducting zone of the respiratory tract [airway generations 0–16 (22)]. In general, the resistance in a given airway of generation i, ri, can be obtained from the equation:
where di, Li, Re and µ, respectively, denote the airway diameter, the airway length, the Reynolds number and the viscosity of air. The formula presumes a fully symmetric lung structure with identical bronchial dimensions within a given airway generation. Replacement of this deterministic architecture by a more realistic stochastic lung structure requires the random selection of di and Li from respective probability density functions computed for each generation of the tracheobronchial tree (23). From a physical point of view those airways belonging to the same generation may be regarded as resistors in parallel (24), so that in an ideally bifurcating lung the total resistance in generation i, Ri, can be computed according to the equation:
For the more realistic case of a stochastic lung architecture, Ri is based on the formula
The RAW of the entire tracheobronchial tree, resting tidal breathing (RTB), was determined by assuming the single airway generations of the lung as resistors in series, for what reason this important parameter corresponds to the sum of resistances computed for the respective generations:
The scaling procedure applied to the diseased lungs consisted of two steps: first, FRC-calibrated lungs were theoretically produced on the basis of physiological data published for 10 patients suffering from chronic bronchitis (21). Hypothetical RAW values were compared with published ones in order to guarantee a certain degree of accuracy. In a second step RTB was computed for the modeled structures by application of Eq. [1-5]. If RTB was smaller than RAW, respective scaling factors were further decreased, and calculations were conducted once more. If, on the other hand, RTB was greater than RAW, scaling factors were slightly increased, and computations were repeated.
In this study three different scaling scenarios were applied: (I) uniform scaling of the entire tracheobronchial tree (model 1); (II) subdivision of the airway system into two anatomically distinct regions [bronchial (BB) and bronchiolar (bb)], whereby bronchial airways were categorized by diameters >0.34 cm and bronchiolar airways by diameters ≤0.34 cm (20), different scaling strategies within the two compartments (model 2); (III) limitation of airway scaling to single lung lobes (model 3). In model 1 all airway diameters of the lung are subjected to a constant reduction of their calibers by using Eq. [1], and the newly generated morphometric data are proven by application of Eq. [2-5]. In the case of model 2 RAWs for the two anatomical regions are calculated separately, thereby using different scaling factors. The total RAW of the tracheobronchial tree, however, has to correspond with respective experimental values. In model 3 the lung structure is subdivided into five lobar parts, within which scaling procedure and computation of RAW is conducted independently according to Eq. [2-5]. Finally, the total RAW must again show a good correspondence with experimental values.
Modeling particle deposition in lungs affected by chronic bronchitis
For the simulation of particle deposition, the computer code IDEAL developed by Koblinger et al. (25) and refined by numerous succeeding studies (26-40) was used. This mathematical approach as founded on the random-walk algorithm, with single particle trajectories running along randomly selected paths through the tubular and alveolar structures. Due to the generation of larger numbers (e.g., 10,000) of such particle trajectories by the application of the Monte Carlo method respective statistical evaluations of the inhalation scenarios can be carried out. Particle transport takes place in a stochastic lung architecture including realistic morphometric data of the tracheobronchial tree and the acinar region (25). For the computation of particle deposition four deposition mechanisms (Brownian motion, inertial impaction, interception, gravitational settling) are distinguished which are expressed in the model by empirical and analytical equations (20,41-54). In general, deposition was predicted for unit-density spheres ranging in diameter from 0.001 to 10 µm. Besides total deposition denoting the entire fraction of particles captured in the lung structures, also regional (i.e., extrathoracic, tubular, alveolar) and local (i.e., airway generation-related) deposition were simulated. For this purpose, two different breathing scenarios [sitting breathing, light-work breathing (20)] were assumed.
Results
Total and regional particle deposition in bronchitis patients
As illustrated in Figures 2 and 3, the theoretical model predicts noticeable differences of particle deposition in patients suffering from chronic bronchitis and particle deposition in healthy controls. The degree of this discrepancy depends on the airway calibration model used for the computations. With regard to total particle deposition spheres ranging in diameter from 0.001 µm to 10 µm are characterized by higher deposition values in bronchitis patients with respect to healthy subjects. Depending on the applied calibration approach differences of total deposition exhibit a variation between 5 and 10% in the case of particle diameters <1 µm. For larger particles, these discrepancies may reach up to 20%. Around a particle diameter of about 0.5 µm deposition shows its minimal values, whereas very small and large particles deposit in the respiratory tract with maximal intensity (Figure 2A). Any change of the breathing conditions produces only insignificant modifications of the deposition curves. Thereby, deposition values of particles ≤1 µm are decreased, whilst respective values of spheres exceeding a diameter of 1 µm are enhanced (Figure 2B).

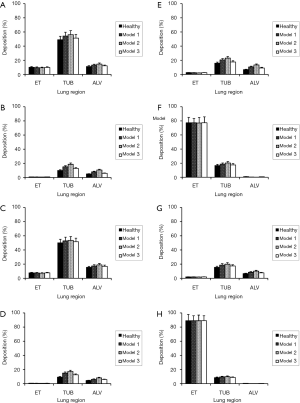
Regional particle deposition was investigated for four different size categories: 0.01, 0.10, 1.0 and 10 µm. Under sitting breathing conditions 0.01 µm particles show an enhanced tubular and alveolar deposition in bronchitis patients compared to healthy controls. A rather similar trend can be recognized for particles with diameters of 0.10 and 1.0 µm, although spheres belonging to these size categories are generally characterized by lower regional deposition quantities. In the case of 10 µm particles preferential deposition occurs in the extrathoracic and tubular region, whereas alveolar particle accumulation reaches a minimum. Disease-related changes of the bronchial architecture have only minor effects on the deposition behavior of inhaled particles (Figure 3A,B,C,D). Modification of the breathing conditions from sitting to light-work inhalation causes only slight changes of the regional deposition fractions. Concretely speaking, smallest particles (0.01 µm) increasingly deposit in the alveoli instead of the tubular structures, whereas largest particles (10 µm) exhibit a further increase of extrathoracic deposition at the cost of tubular accumulation (Figure 3E,F,G,H).
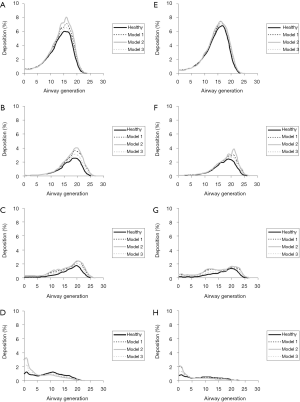
Generation-by-generation deposition of particles in bronchitis patients
Deposition of spherical particles (0.01, 0.10, 1.0, 10 µm) in single airway generations [0 (trachea) to 30 (respiratory bronchiole)] is characterized by typical patterns for both sitting and light-work breathing. Under sitting inhalation 0.01 µm spheres have their maximal deposition in airway generation 17, whilst 0.10 and 1.0 µm particles mainly deposit in airway generation 20. The percentage fraction of accumulated particulate mass remarkably decreases with increasing particle diameter. Any bronchitis-induced recalibration of the airways causes an enhancement of local deposition. In the case of particles with a diameter of 10 µm maximal deposition takes place in the first two airway generations, whereas deposition in the following generations is subject to a continuous decline. Reduction of the airway calibers results in an amplification of this effect (Figure 4A,B,C,D). Under light-work inhalation deposition maxima of 0.01 µm spheres are slightly increased, whereas those of the other particle categories are decreased. The last effect mainly occurs for 10 µm particles, from which only few percent are able to penetrate into the thoracic compartment of the respiratory tract. Generally, discrepancies in local deposition between bronchitis patients and healthy controls become smaller (Figure 4E,F,G,H).
Discussion
Chronic bronchitis is marked by partly significant changes of the tracheobronchial architecture, whereby continuous narrowing of the airway calibers due to edema, inflammatory processes and mucus hypersecretion become most apparent (1-4). Reduction of the air volume in the bronchial compartment results in an increase of the volume distal to the terminal bronchioles. At the end of this process stands the manifestation of a specific type of emphysema, where the alveoli are abnormally inflated due to their loss of elasticity and the disintegration of alveolar septa (5-7). Deposition of particles inhaled into such a modified respiratory system clearly differs from that occurring in a healthy lung, whereby theoretical models like that introduced in this contribution can help to appropriately evaluate the differences of intrapulmonary particle behaviour. Previous studies have already yielded evidence that deposition of particles with different sizes and shapes is controlled to a high degree by bronchial and alveolar morphometry (26-40).
According to the model predictions presented here total particle deposition is generally increased in bronchitis patients with respect to healthy controls. Respective differences of the deposition values are on the order of 5% to 20% and among other depend on the particle diameter and the intensity of aerosol inhalation. As found in earlier studies all known deposition mechanisms exhibit a certain dependence on airway morphometry in so far as smaller airway calibers cause higher deposition rates (20,26,41-54). Any increase of the inhalation flow rate has two main consequences: (I) particles driven by Brownian motion get a higher chance to run through the airway generations and to be subsequently exhaled, so that total deposition is subject to a slight decrease (26-30); (II) particles seized by inertial impaction, interception and gravitational settling are either increasingly deposited in the uppermost airway generations or undergo enhanced deposition in the peripheral structures, so that total deposition is remarkably amplified (26-30).
The phenomena reported for total deposition are also confirmed by the model predictions with regard to regional deposition. Independent of the used scaling model chronic bronchitis leads to an increase of tubular and alveolar particle accumulation. This effect can be mostly recognized for particles ranging in size from 0.01 to 1.0 µm. Larger particles, however, are already filtered out in the extrathoracic and uppermost tracheobronchial airways, so that disease-induced deposition changes in more distal airway generations are of minor significance (20,25-30). Basically, bronchitis patients exhibit an enhanced bronchial filtering capacity of µm-sized particles, whilst spheres belonging to the nm-scale have a higher probability to reach the distal (= alveolar) lung structures (26-30).
With regard to local particle deposition the theoretical observations discussed above find their broad confirmation. Small and intermediately sized spheres are maximally deposited in airway generations 17 to 20, whereby the intensity of accumulation is additionally increased in bronchitis patients. Large particles (10 µm), on the other hand, are already filtered out in the most proximal airway generations. The shift of the deposition maximum from 0.01 to 0.1 and 1.0 µm particles can be explained by the size-related switch from diffusion-induced deposition to mass-induced deposition. In the latter case inhaled particles are enabled to surmount longer intrapulmonary transport distances (20,25,30-54).
Conclusions
Based on comprehensive model computations it can be concluded that total, regional and local deposition patterns predicted for patients with chronic bronchitis clearly differ from those patterns calculated for healthy subjects. In general, bronchial deposition undergoes a measureable enhancement in obstructed airways which may be of increased interest in association with inhalation therapies. These procedures try to obtain maximal particle deposition in the respective target regions, whereby this aim is either fulfilled by variation of particle sizes (shapes) or attained by a change of the inhalation flow rate. Theoretical models can offer valuable assistance for an optimization of these frame conditions. Another aspect concerns the diagnosis of disease grade (= grade of airway obstruction) which can be also supported by hypothetical computations. The related potential of the model will be clarified in future studies.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2019.03.03). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet 2012;379:1341-51. [Crossref] [PubMed]
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Respirology 2017;22:575-601. [Crossref] [PubMed]
- National Institute for Health and Clinical Excellence. Clinical guideline 101: chronic obstructive pulmonary disease. London: NIHCE, 2010.
- Barnes PJ. Mechanisms in COPD: differences from asthma. Chest 2000;117:10S-4S. [Crossref] [PubMed]
- Ward H, Toledano MB, Shaddick G, et al. Oxford handbook of epidemiology for clinicians. Oxford: Oxford University Press, 2012.
- Barnes PJ, Drazen JM, Rennard SI, et al. Asthma and COPD: basic mechanisms and clinical management. San Diego: Academic Press, 2009.
- Palange P, Simonds AK. editors. ERS Handbook of Respiratory Medicine. Sheffield: European Respiratory Society, 2013.
- Currie GP. editor. ABC of COPD. London: Wiley-Blackwell, 2010.
- Mackay AJ, Hurst JR. COPD exacerbations: causes, prevention, and treatment. Med. Clin. N. Am. 2012;96:789-809. [Crossref] [PubMed]
- Chapman S, Robinson G, Stradling J, et al. Oxford handbook of respiratory medicine. Oxford: Oxford University Press, 2009.
- Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;9:CD002309 [PubMed]
- Tønnesen P. Smoking cessation and COPD. Eur Respir Rev 2013;22:37-43. [Crossref] [PubMed]
- Cave AC, Hurst MM. The use of long acting ß2-agonists, alone or in combination with inhaled corticosteroids, in chronic obstructive pulmonary disease (COPD): a risk-benefit analysis. Pharmacol Ther 2011;130:114-43. [Crossref] [PubMed]
- Vestbo J, Sorensen T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999;353:1819-23. [Crossref] [PubMed]
- Burge PS, Calverley PMA, Jones PW, et al. Randomised, double-blind, placebo-controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000;320:1297-303. [Crossref] [PubMed]
- Niewoehner DE, Erbland ML, Deupree RA, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. New Engl J Med 1999;340:1941-47. [Crossref] [PubMed]
- Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456-60. [Crossref] [PubMed]
- Hughes JMB, Hoppin FG, Mead J. Effect of lung inflation on bronchial length and diameter in excised lungs. J Appl Physiol 1972;32:25-35. [Crossref] [PubMed]
- Phalen RF, Oldham MJ, Beaucage CB, et al. Postnatal enlargement of human tracheobronchial airways and implications for particle deposition. Anat Rec 1985;212:368-80. [Crossref] [PubMed]
- International Commission on Radiological Protection (ICRP). Human respiratory tract model for radiological protection. Publication 66. Oxford: Pergamon Press, 1994.
- Kim CS, Kang TC. Comparative measurement of lung deposition of inhaled fine particles in normal subjects and patients with obstructive airway disease. Am J Resp Crit Care Med 1997;155:899-905. [Crossref] [PubMed]
- Pedley TJ, Schroter RC, Sudlow MF. The prediction of pressure drop and variation of resistance in the human bronchial airways. Respir Physiol 1970;9:387-405. [Crossref] [PubMed]
- Koblinger L, Hofmann W. Analysis of human lung morphometric data for stochastic aerosol deposition calculations. Phys Med Biol 1985;30:541-56. [Crossref] [PubMed]
- Kim CS, Brown LK, Lewards GG, et al. Deposition of aerosol particles and flow resistance in mathematical and experimental airway models. J Appl Physiol Respir Environ Exerc Physiol 1982;55:154-63. [PubMed]
- Koblinger L, Hofmann W. Monte Carlo modeling of aerosol deposition in human lungs. Part I: Simulation of particle transport in a stochastic lung structure. J Aerosol Sci 1990;21:661-74. [Crossref]
- Sturm R. Theoretical deposition of nanotubes in the respiratory tract of children and adults. Ann Transl Med 2014;2:6. [PubMed]
- Sturm R. Nanotubes in the respiratory tract - Deposition modeling. Z med Phys 2015;25:135-45. [Crossref] [PubMed]
- Sturm R. Inhaled nanoparticles. Phys Today 2016;69:70. [Crossref]
- Sturm R. A stochastic model of carbon nanotube deposition in the airways and alveoli of the human respiratory tract. Inhal Toxicol 2016;28:49-60. [Crossref] [PubMed]
- Sturm R. Theoretical deposition of carcinogenic particle aggregates in the upper respiratory tract. Ann Transl Med 2013;1:25. [PubMed]
- Sturm R. Spatial visualization of theoretical nanoparticle deposition in the human respiratory tract. Ann Transl Med 2015;3:326. [PubMed]
- Sturm R. Deposition of ultrafine particles with various shapes in the human alveoli – a model approach. Comp Math Biol 2016;5:4.
- Sturm R. A theoretical approach to the deposition of cancer-inducing asbestos fibers in the human respiratory tract. TOLCJ 2009;2:1-11. [Crossref]
- Sturm R, Hofmann W. Modellrechnungen zur Deposition nicht-sphärischer Teilchen in den oberen Luftwegen der menschlichen Lunge. Z Med Phys 2009;19:38-46. [Crossref] [PubMed]
- Sturm R. Theoretical models of carcinogenic particle deposition and clearance in children’s lungs. J Thorac Dis 2012;4:368-76. [PubMed]
- Sturm R, Hofmann W. A theoretical approach to the deposition and clearance of fibers with variable size in the human respiratory tract. J Hazard Mater 2009;170:210-8. [Crossref] [PubMed]
- Hofmann W, Sturm R, Winkler-Heil R, et al. Stochastic model of ultrafine particle deposition and clearance in the human respiratory tract. Radiat Prot Dosimetry 2003;105:77-80. [Crossref] [PubMed]
- Sturm R, Hofmann W. Stochastic model for the spatial visualization of particle-deposition patterns in the lung and their significance in lung medicine. Z Med Phys 2006;16:140-7. [Crossref] [PubMed]
- Sturm R. A computer model for the simulation of nonspherical particle dynamics in the human respiratory tract. Phys Res Int 2012;1-11. [Crossref]
- Sturm R, Hofmann W 3D. -Visualization of particle deposition patterns in the human lung generated by Monte Carlo modeling: methodology and applications. Comput Biol Med 2005;35:41-56. [Crossref] [PubMed]
- Sturm R. Theoretical and experimental approaches to the deposition and clearance of ultrafine carcinogens in the human respiratory tract. Thorac Cancer 2011;2:61-8. [Crossref] [PubMed]
- Sturm R. Theoretical models of carcinogenic particle deposition and clearance in children’s lungs. J Thorac Dis 2012;4:368-76. [PubMed]
- Sturm R. A computer model for the simulation of fiber-cell interaction in the alveolar region of the respiratory tract. Comput Biol Med 2011;41:565-73. [Crossref] [PubMed]
- Sturm R. Theoretical models for dynamic shape factors and lung deposition of small particle aggregates originating from combustion processes. Z med Phys 2010;20:226-34. [Crossref] [PubMed]
- Hofmann W, Bolt L, Sturm R, et al. Simulation of three-dimensional particle deposition patterns in human lungs and comparison with experimental SPECT data. Aerosol Sci Technol 2005;39:771-81. [Crossref]
- Sturm R, Hofmann W. A computer program for the simulation of fiber deposition in the human respiratory tract. Comput Biol Med 2006;36:1252-67. [Crossref] [PubMed]
- Sturm R. Theoretical approach to the hit probability of lung-cancer-sensitive epithelial cells by mineral fibers with various aspect ratios. Thoracic Cancer 2010;1:116-25. [Crossref] [PubMed]
- Sturm R. A three-dimensional model of tracheobronchial particle distribution during mucociliary clearance in the human respiratory tract. Z med Phys 2013;23:111-9. [Crossref] [PubMed]
- Sturm R. Computer-aided generation and lung deposition modeling of nano-scale particle aggregates. Inhal Toxicol 2017;29:160-8. [Crossref] [PubMed]
- Sturm R. Modeling the deposition of bioaerosols with variable size and shape in the human respiratory tract–a review. J Adv Res 2012;3:295-304. [Crossref]
- Sturm R. Inhalation of nanoplatelets - Theoretical deposition simulations. Z Med Phys 2017;27:274-84. [Crossref] [PubMed]
- Sturm R. Clearance of carbon nanotubes in the human respiratory tract-a theoretical approach. Ann Transl Med 2014;2:46. [PubMed]
- Sturm R. Theoretical models for the simulation of particle deposition and tracheobronchial clearance in lungs of patients with chronic bronchitis. Ann Transl Med 2013;1:3. [PubMed]
- Sturm R. Modeling the delay of mucous flow at the carinal ridges of the human tracheobronchial tree. Comp Math Biol 2014;3:6.
Cite this article as: Sturm R. Particles in the lungs of patients with chronic bronchitis—part 1: deposition modeling. J Public Health Emerg 2019;3:5.


