
Theoretical model of clearance in the tracheobronchial airways of healthy subjects and smokers
Introduction
Previous investigations came to the consistent result that particulate mass deposited in the tracheobronchial tree is cleared by different mechanisms. Besides the so-called mucociliary escalator, representing the fast clearance phase, also much slower processes such as particle transcytosis, intra- and extracellular particle storage as well as particle uptake by macrophages may be observed (1-12). According to highly specific clearance experiments fast transport of particulate mass on the airway epithelium is mainly completed within the first 24 h after aerosol exposure. Slow bronchial clearance, on the other hand, is characterized by half-times varying between 5 and 20 d, so that particles undergoing this process are completely removed from the airway structures within 25 to 100 d (13-15). Other studies could find out that total duration of tracheobronchial clearance exhibits a significant dependence on several physical and physiological factors, among which particle traits and breathing conditions may be evaluated as most essential (16-18). All these factors determine the deposition sites and slow clearance fractions of the inhaled particulate substances and thus have a remarkable effect on particle retention in the airways (19,20).
Based upon a multitude of medical investigations cigarette smoking has to be categorized as severe cause of non-malignant diseases including COPD, chronic bronchitis, emphysema, and asthma-related symptoms. All insufficiencies mentioned above imply a continuous decline in lung function and related respiratory dysplasia (coughing, phlegm, wheezing, dyspnoea) (21,22). From a physical point of view, cigarette smoke can be classified as aerosol with solid particles being suspended in a gaseous phase. Both solid and gaseous components of the smoke aerosol consist of thousands of chemical compounds, with numerous species of this mixture acting as toxins or carcinogens (23). Many ingredients of cigarette smoke have the potential to likewise injure the bronchial and alveolar structures through a variety of mechanisms. Some compounds perform adverse effects on host defenses such as the clearance system, whereas others act through specific or non-specific mechanisms. Formaldehyde and acrolein, for instance, may be regarded as cilia-toxic substances, thereby wielding a negative effect on cilia motion and, thus, impairing the innate lung defenses (23). Carbon monoxide (CO), nitrogen dioxide (NO2) and several metals (e.g., Cd) disturb the intracellular oxidant activity or become targets of regulatory processes due to their toxic properties (23-25).
Interference of the innate lung defense system by the inhalation of cigarette smoke is expressed by (I) a permanent increase of mucus production and (II) a continuous decrease of clearance efficiency in the bronchial and alveolar compartments (26). Significant weakening of the defenses mentioned above, however, results in a partly dramatic enhancement of the infection risk (27,28). Besides the superficial effects summarized above, cigarette smoke also disposes of the capacity to disrupt tight junctions forming the epithelial barrier (29,30). In addition, it may initiate the infiltration of the damaged tissue by a great number of inflammatory immune cells (23). Any increase in microvascular permeability facilitates the leakage of large fibrinogen molecules from the vessels and the subsequent formation of new tissue, resulting in a remodeling of the bronchial and alveolar walls. In small ciliated and non-ciliated airways this process induces a systematic thickening of epithelial and subepithelial cell layers (fibrosis) and therefore a reduction of the inner diameters (Figure 1) (31).
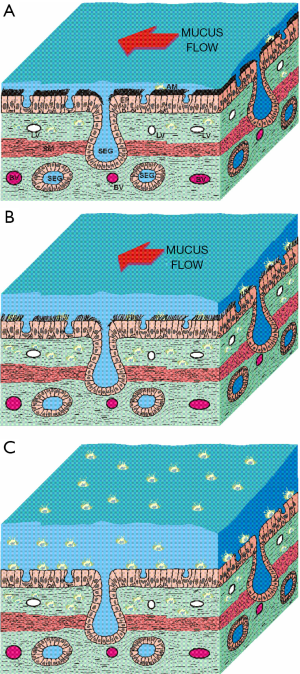
Meanwhile, interference of the lungs by inhaled cigarette smoke may be regarded as well-documented phenomenon. However, particle deposition in the lungs of occasional and heavy smokers is much better understood than clearance processes acting upon the deposited particulate mass (32,33). Since phenomena of smoke-cell interaction were comprehensively illuminated during the past years (23), new leverage points for specific clearance models dealing with particle removal in healthy and diseased lungs were created. The present contribution pursues two objectives: besides the description of a highly validated mathematical approach of tracheobronchial clearance in healthy subjects and smokers (34-38), also preliminary simulation results describing the clearance scenarios of specific particles (1 µm) are presented.
Methods
Model description
As proposed in previous studies (34-38) tracheobronchial particle clearance can be well simulated by a multi-compartment model. This theoretical approach provides the consideration of all cellular and non-cellular units of the airway epithelium which bear the capability of particle uptake and storage. Concretely speaking, the tracheobronchial region of the healthy lung includes four such units that can be treated as separate model compartments. Particles entering the tracheobronchial tree usually deposit on the highly viscous gel layer of the airway mucus (compartment 1). This cilia-driven blanket is transported towards the trachea, where it is either swallowed or expectorated. Some particles penetrate the mucus at thinned sites or pass this layer via specific discontinuities (10-12), which results in their temporary storage in the periciliary liquid (Sol layer, compartment 2). Particulate mass escaping from mucociliary clearance is mainly subjected to the uptake by airway macrophages (compartment 4) or epithelial cells (compartment 5). In the theoretical model, the gastrointestinal tract and the lymphatic/vascular system are defined as two further units, within which terminal storage of particulate mass takes place (Figure 2A).
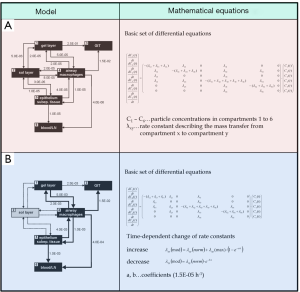
The model compartments introduced above are connected by specific rate constants (transfer rates) that express the velocity and, therefore, effectiveness of mass transfer between the single storage units. Mucociliary clearance has to be understood as mass transfer between the gel layer (compartment 1) and the gastrointestinal tract (compartment 3). In healthy lungs, this process is commonly characterized by rather high rate constants. Transepithelial particle transport (compartment 2 → compartment 5 → compartment 6) or clearance by airway macrophages (compartment 1/2 → compartment 4), on the other hand, is marked by low rate constants. Except for the final accumulation units (compartment 3 and 6), mass transfer between single units as assumed to occur in both directions (34,35). Those particles reaching the periciliary liquid may be transferred back on the gel layer with the help of extensive cilia beating. Particulate mass, which has been already phagocytosed by macrophages, can be again transported to other compartments after the death of the phagocytic cells. Basic mathematical equations standing behind the multi-compartment model are also summarized in Figure 2A.
The modified model describing tracheobronchial clearance in the lungs of smokers exhibits some remarkable changes with respect to the basic approach outlined above. As illustrated in Figure 1, the periciliary liquid is marked by its successive disappearance in diseased airways, so that the related model compartment only plays a marginal role. Additionally, several rate constants undergo a specific temporal change: Whilst mass transfer between the gel layer and the gastrointestinal tract becomes continuously retarded in smoker lungs, airway macrophages and epithelial cells gain enhanced importance and execute an accelerated uptake of particulate mass. Mathematical equations standing behind the modified approach are, for the sake of brevity, summarized in Figure 2B.
Model setup
For preliminary model predictions presented in this contribution spherical particles with a uniform diameter of 1 µm and unit-density (1 g·cm−3) were used. Inhalation of the particle-loaded aerosol was supposed to take place by a respiratory system of average size (FRC =3,300 mL) under sitting breathing conditions (31). Such frame conditions, however, result in an average lung deposition of 18%, whereby two third of all particles deposited in the bronchial structures are subjected to slow clearance processes (10-12). The remaining third undergoes fast mucociliary clearance. Bronchial clearance in healthy and diseased lungs was predicted for a time interval of 10 d, because crucial processes take place within this temporal window.
Results
As illustrated in Figure 3, particle concentrations predicted for the single compartments of the model are characterized by time-dependent changes. Regarding tracheobronchial clearance of healthy subjects highest efficiency can be determined for mucociliary transport. In addition, all particulate mass captured in the periciliary liquid (= sol layer) is effectively removed within a rather short period of time. Concretely speaking, particle clearance on the mucus layer immediately starts after aerosol exposure and covers a period of several hours. According to the theoretical approach the sol layer is completely cleared from any particulate substances after 6 d. In healthy subjects the gastrointestinal tract commonly acts as main particle accumulation compartment, so that 60% of the deposited mass are transported to this unit within 2 d. After mucociliary clearance has completed its main phase, particles are increasingly cleared via airway macrophages and the bronchial epithelium. Three days after aerosol exposure capture of particulate mass by airway macrophages performs a maximum, whilst transepithelial clearance already reaches its peak 1.5 d after the inhalation event. After 10 d, particle concentrations in macrophages and epithelial cells are declined to few percent, whereas concentrations of particulate mass in the gastrointestinal tract and lymphatic/vascular system are increased to 88% and 5%, respectively (Figure 3A).
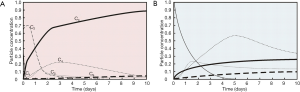
In the lungs of smokers, mucociliary particle clearance is commonly characterized by a retardation covering one order of magnitude (Figure 3B). Removal of particulate mass by airway macrophages and epithelial transcytosis, on the other hand, significantly gains in importance. Therefore, macrophages are able to absorb a maximal particle mass of 57% (5 d after exposure), whilst the epithelium accumulates 23% (30 h after exposure) of all particles deposited in the bronchial airways. After 10 d, only 25% of the particulate mass have reached the gastrointestinal tract, whereas 11% of the particles have been transported to the lymphatic and vascular system. The remaining mass is still retained in the bronchial airways and may interact with diverse cells of the airway wall.
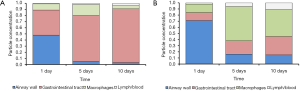
Main compartments factored into the bronchial clearance process of healthy and diseased lungs are summarized in the bar charts of Figure 4. In healthy controls, the gastrointestinal tract is successively supplied with particulate mass, so that 88% of all particles are stored in this unit after 10 d. The remaining particles have been either captured by macrophages or transported to the lymphatic/vascular system. In heavy smokers, importance of the gastrointestinal tract as final accumulation compartment is continuously declined. After 10 d, only 25% of all particles have reached this unit, whereas 75% have remained in the lungs or their direct vicinity. An essential role is attributed to macrophages, which mainly transport the captured particles towards the lymphatic system.
Discussion and conclusions
According to the theoretical results presented in this contribution bronchial clearance predicted for heavy smokers remarkably deviates from that observed for healthy controls. This difference is expressed in several respects: (I) in lungs injured by cigarette smoke clearance of deposited particles from the tracheobronchial structures is significantly retarded with respect to the healthy respiratory tract. Concretely speaking, more than 50% of the total particulate mass are still retained in the airways after 10 d (10% in healthy controls); (II) in heavy smokers fast clearance mechanisms such as the mucociliary escalator are successively superseded by much slower processes with prolonged interaction between deposited particulate mass and lung tissues being susceptible to inflammatory reactions or malignant transformations; (III) in diseased lungs the lymphatic and vascular system increasingly occur as particle accumulation compartments, thereby continuously taking over the role of the gastrointestinal tract in healthy subjects.
All changes of bronchial clearance noted above have to be regarded as result of an extensive impairment of the cilia beating activity by cilia-toxic substances (23-25). In addition, an enhanced release of mucus and inflammatory cells into the airway lumen can be recognized (29-31). Medical and histological studies (23,31) categorize ciliated cell destruction as well as paralysis of single cilia as rather protracted processes, whereby small ciliated airways represent the preferential target sites of inhaled smoke particles with a size varying between 100 and 300 nm (32,39-43). Significant percentages of the inspired particles are subjected to electrostatic aggregation or hydrophilic growth, finally resulting in sizes larger than 1 µm (32). Such aggregates are either deposited in the upper bronchial airways after fast breathing or reach the central airways after slow inhalation of the cigarette aerosol (39-49). It has to be noted in this context that complete interruption of mucociliary particle clearance requires the permanent burdening of the lung with cigarette smoke over an extended period of time.
The enhanced clearance of particles through the bronchial epithelium and subepithelial cell layers in smoker lungs is commonly associated with severe health effects: Due to a considerable limitation of the absorption capacity of the lymphatic and vascular system (see low rate constants in Figure 2), particulate masses undergo temporal storage in the epithelial and subepithelial cells included in the slow clearance process. In these cellular units, however, metabolic processes may be affected by highly insoluble ingredients of the cigarette smoke, so that biosynthetic and lytic cycles become severely disturbed. Further deposition of smoke particles in the lung airways may cause an overload of bronchial cells and, as a consequence of that, their complete dysfunction (23,31,33). The enhanced impairment of the bronchial tissues is usually accompanied by a higher susceptibility of these sites for infections with the successive development of chronic airway diseases and related deuteropathy (bronchiectasis, emphysema) (23,46-49).
Meanwhile, main processes with regard to the effect of cigarette smoke on various lung tissues are well understood. Nevertheless, numerous phenomena coming off on the molecular level still bear some open questions and, thus, have to be subjected to more detailed investigations in future. Here the present model with its multitude of particle transport routes can offer valuable assistance.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2018.03.04). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Albert RE, Arnett LC. Clearance of radioactive dust from the human lung. AMA Arch Ind Health 1955;12:99-106. [PubMed]
- Albert RE, Lippmann M, Briscoe W. The characteristics of bronchial clearance in humans and the effects of cigarette smoking. Arch Environ Health 1969;18:738-55. [Crossref] [PubMed]
- Albert RE, Lippmann M, Peterson HT Jr, et al. Bronchial deposition and clearance of aerosols. Arch Intern Med 1973;131:115-27. [Crossref] [PubMed]
- Lippmann M, Yeates DB, Albert RE. Deposition, retention, and clearance of inhaled particles. Br J Ind Med 1980;37:337-62. [PubMed]
- Brain JD, Proctor D, Reidl LM. Respiratory defense mechanisms. New York: Marcel Dekker Inc., 1977.
- Wolff RK. Mucociliary Function. In Parent RA. editor. Comparative Biology of the Normal Lung. New York: CRC Press, 1989:659-80.
- Oberdörster G. Lung clearance of inhaled insoluble and soluble particles. J Aerosol Med 1988;1:289-330. [Crossref]
- Hofmann W, Sturm R, Asgharian B. Stochastic simulation of particle clearance in human bronchial airways. J Aerosol Sci 2001;32:S807-8.
- Sturm R, Hofmann W, Scheuch G, et al. Particle clearance in human bronchial airways: Comparison of stochastic model predictions with experimental data. Ann Occup Hyg 2002;46:329-33. [PubMed]
- Sturm R, Hofmann W. Mechanistic interpretation of the slow bronchial clearance phase. Radiat Prot Dosimetry 2003;105:101-4. [Crossref] [PubMed]
- Hofmann W, Sturm R. Stochastic model of particle clearance in human bronchial airways. J Aerosol Med 2004;17:73-89. [Crossref] [PubMed]
- Sturm R, Hofmann W. Stochastic modeling predictions for the clearance of insoluble particles from the tracheobronchial tree of the human lung. Bull Math Biol 2007;69:395-415. [Crossref] [PubMed]
- Stahlhofen W. Human lung clearance following bolus inhalation of radioaerosols. In: Crapo JD, Smolko ED, Miller FJ, et al. editors. Extrapolation of dosimetric relationships for inhaled particles and gases. San Diego: Academic Press, 1989:153-66.
- Stahlhofen W, Koebrich R, Rudolf G, et al. Short-term and long-term clearance of particles from the upper human respiratory tract as a function of particle size. J Aerosol Sci 1990;21:S407-10. [Crossref]
- Scheuch G, Kreyling W, Haas F, et al. The clearance of polystyrene particles from UO2 fuel element fabrication. Health Phys 1993;48:29-48.
- Philipson K, Falk R, Svartengren M, et al. Does lung retention of inhaled particles depend on their geometric diameter? Exp Lung Res 2000;26:437-55. [Crossref] [PubMed]
- Sturm R, Hofmann W. A computer program for the simulation of fiber deposition in the human respiratory tract. Comput Biol Med 2006;36:1252-67. [Crossref] [PubMed]
- Sturm R. Theoretical models for dynamic shape factors and lung deposition of small particle aggregates originating from combustion processes. Z med Phys 2010;20:226-34. [Crossref] [PubMed]
- Hofmann W, Sturm R, Winkler-Heil R, et al. Stochastic model of ultrafine particle deposition and clearance in the human respiratory tract. Radiat Prot Dosimetry 2003;105:77-9. [Crossref] [PubMed]
- Sturm R. Deposition and cellular interaction of cancer-inducing particles in the human respiratory tract: Theoretical approaches and experimental data. Thorac Cancer 2010;1:141-52. [Crossref] [PubMed]
- Mason RJ, Broaddus VC, Murray JF, et al. Murray and Naddel’s Textbook of Respiratory Medicine. 4th ed. St. Louis: Elsevier, 2005.
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US), 2010.
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco Smoke and Involuntary Smoking. Vol. 83. Lyon: IARC, 2004.
- Gardner DE, Crapo JD, McClellan RO. Toxicology of the Lung. 3rd ed. Philadelphia: Taylor & Francis, 2000.
- Klaassen CD. editor. Casarett and Doull’s Toxicology: The Basic Science of Poisons. 6th ed. New York: McGraw-Hill, 2001.
- Hogg JC. Lung structure and function in COPD. Int J Tuberc Lung Dis 2008;12:467-79. [PubMed]
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 2002;109:571-7. [Crossref] [PubMed]
- Drannik AC, Pouladi MA, Robbins CS, et al. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am J Resp Crit Care Med 2004;170:1164-71. [Crossref] [PubMed]
- Jones JG, Minty BD, Lawler P, et al. Increased alveolar epithelial permeability in cigarette smokers. Lancet 1980;315:66-8. [Crossref] [PubMed]
- Hulbert WC, Walker DC, Jackson A, et al. Airway permeability to horseradish peroxidase in guinea pigs: the repair phase after injury by cigarette smoke. Am Rev Respir Dis 1981;123:320-6. [PubMed]
- Svartengren M, Svartengren K, Europe E, et al. Long-term clearance from small airways in patients with chronic bronchitis: experimental and theoretical data. Exp Lung Res 2004;30:333-53. [Crossref] [PubMed]
- International Commission on Radiological Protection (ICRP). Human Respiratory Tract Model for Radiological Protection, Publication 66. Oxford: Pergamon Press, 1994.
- Sturm R. A computer model for the simulation of fiber-cell interaction in the alveolar region of the respiratory tract. Comput Biol Med 2011;41:565-73. [Crossref] [PubMed]
- Sturm R, Hofmann W. A multi-compartment model for slow bronchial clearance of insoluble particles - extension of the ICRP human respiratory tract models. Radiat Prot Dosimetry 2006;118:384-94. [Crossref] [PubMed]
- Sturm R. A computer model for the clearance of insoluble particles from the tracheobronchial tree of the human lung. Comput Biol Med 2007;37:680-90. [Crossref] [PubMed]
- Sturm R. Age-dependence and intersubject variability of tracheobronchial particle clearance. Pneumon 2011;24:77-84.
- Sturm R. A three-dimensional model of tracheobronchial particle distribution during mucociliary clearance in the human respiratory tract. Z med Phys 2013;23:111-9. [Crossref] [PubMed]
- Sturm R. Clearance of carbon nanotubes in the human respiratory tract – a theoretical approach. Ann Transl Med 2014;2:46. [PubMed]
- Sturm R. Theoretical approach to the hit probability of lung-cancer-sensitive epithelial cells by mineral fibers with various aspect ratios. Thorac Cancer 2010;1:116-25. [Crossref] [PubMed]
- Sturm R. Theoretical and experimental approaches to the deposition and clearance of ultrafine carcinogens in the human respiratory tract. Thorac Cancer 2011;2:61-8. [Crossref] [PubMed]
- Sturm R. Theoretical models of carcinogenic particle deposition and clearance in children’s lungs. J Thorac Dis 2012;4:368-76. [PubMed]
- Sturm R. A computer model for the simulation of nonspherical particle dynamics in the human respiratory tract. Phys Res Intern 2012. doi:
10.1155/2012/142756 . - Sturm R. Theoretical deposition of nanotubes in the respiratory tract of children and adults. Ann Transl Med 2014;2:6. [PubMed]
- Sturm R. Nanotubes in the respiratory tract - Deposition modeling. Z med Phys 2015;25:135-45. [Crossref] [PubMed]
- Sturm R. Spatial visualization of nanoparticle deposition in the human respiratory tract. Ann Transl Med 2015;3:21. [PubMed]
- Sturm R, Hofmann W. Stochastic simulation of alveolar particle deposition in lungs affected by different types of emphysema. J Aerosol Med 2004;17:357-72. [Crossref] [PubMed]
- Sturm R. Carbon nanotubes in the human respiratory tract – clearance modeling. Ann Work Expo Health 2017;61:226-36. [Crossref] [PubMed]
- Sturm R. Computer-aided generation and lung deposition modeling of nano-scale particle aggregates. Inhal Toxicol 2017;29:160-8. [Crossref] [PubMed]
- Sturm R. Inhalation of nanoplatelets – theoretical deposition simulations. Z med Phys 2017;27:274-84. [Crossref] [PubMed]
Cite this article as: Sturm R. Theoretical model of clearance in the tracheobronchial airways of healthy subjects and smokers. J Public Health Emerg 2018;2:13.


