
Theoretical simulation of diesel exhaust particle clearance from the human respiratory tract
Introduction
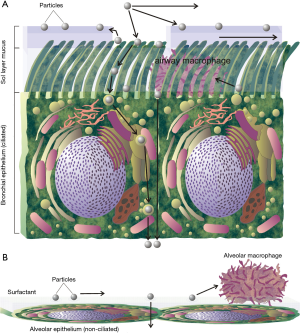
Since the pioneering studies of Albert and coworkers (1-3) it has been considered as a well-known fact that particles deposited in the tracheobronchial airways undergo a fast clearance process, which is mostly completed 24 h after exposure. In the 1980s it could be found out that this fast particle removal from the bronchial and bronchiolar structures is based upon the so-called mucociliary escalator (4), where particulate mass captured on a mucus layer is transported towards the trachea due to the beating activity of ciliated cells (5,6). Furthermore, numerous experimental studies could provide evidence for the existence of a slow bronchial clearance phase (7-10), which is characterized by half-times of several days to weeks (11-15) and thus has an efficiency comparable to that of the alveolar clearance phase (14,15). In the meantime, it has to be regarded as a proven fact that both bronchial and alveolar clearance distinguish themselves by a multitude of separate mechanisms, which among other depend on the size of deposited particles and dispose of different velocities (Figure 1) (11-17).
As outlined in previous studies (18-20), diesel exhaust particles (DEP) commonly form irregularly shaped aggregates consisting of small spherical or close-to-spherical components (diameter: 1–10 nm) and measuring 40 to 250 nm in size. In general, aerodynamics of such particles are highly affected by diffusive processes, which induce fast axial and radial spreading of DEP and their enhanced deposition in the upper and central airways of the respiratory tract (21). With regard to the clearance of DEP from proximal and peripheral lung structures both experimental and theoretical investigations (7-17,22) recommend a significant intensification of transepithelial transport processes, causing an increased transfer of particulate mass towards lung-associated lymph nodes and blood capillaries. Recently, it could be demonstrated that extremely small particles (<10 nm) may pass the epithelial barrier through the extracellular matrix within several minutes (23,24), whereas larger particles follow a regular path of transcytosis and may be stored in epithelial cells for an undefined period of time (15-17).
Theoretical models dealing with the clearance of particulate mass from the respiratory tract retrospect on a long developmental process, which took its start in the 1970s (25) and has been characterized by numerous progresses since then (26,27). Modern mathematical approximations of the clearance process include the entirety of particle transport routes occurring in the different lung regions and interpret all these transfer phenomena as multivariate events, which are mostly founded upon stochastic considerations. This means that transport routes and related clearance times are modeled with the help of the principle of contingency (26-33). Finally, comprehensive validation procedures, where available experimental data were compared with related theoretical results, underlined the high predictive accuracy of such probability-based clearance approaches (29,30).
In the present contribution, clearance of DEP deposited in the respiratory tract is simulated in detail, thereby trying to find out eventual particle-related specificities with respect to smaller or larger particulate substances. Modeling results are compared with respective data outlined in earlier studies. The investigation may be regarded as innovative in several respects:
(I) Although clearance of ultrafine particles has already stood in the focus of several experimental and theoretical works (21-23), description of DEP removal from diverse lung regions is limited to a few research papers hitherto (31);
(II) Diesel engine vehicles are still most essential components of urban and rural traffic, so that partly high concentrations of DEP are released into the ambient atmosphere. Therefore, any increase in knowledge with regard to this particle class is highly welcome;
(III) Based upon an exact temporal characterization of single clearance mechanisms hazardous potentials associated with DEP can be evaluated with higher accuracy.
Methods
Brief description of the basic clearance model
The mathematical approach used for the theoretical evaluation of DEP clearance from the respiratory tract has been already outlined in previous contributions (14-18), so that only the most salient features of this predictive tool will be described here. In principle, the clearance model divides the lung into two main compartments, namely (I) the bronchial and bronchiolar airways and (II) the alveolar region. Both parts are characterized by very specific clearance processes, which are summarized in Figure 1. In the upper and central airways of the tracheobronchial tree, particles deposited on a mucus layer are transferred towards the trachea by the mucociliary escalator. That particulate mass, which does not hit the mucus blanket, is stored in the periciliary sol layer, from where it is either transported back on the mucus or taken up by epithelial cells. Particles endocytosed by ciliated or non-ciliated cells commonly undergo an extensive process of transcytosis, at the end of which stands their accumulation in the subepithelial tissues, lymph vessels, and blood capillaries. Particulate mass, which is neither seized by mucociliary clearance nor subjected to the transepithelial route, represents a preferential target of airway macrophages probing the epithelial walls for all possible foreign objects.
In the small peripheral airways (terminal and respiratory bronchioles) and alveoli the mucus blanket is substituted by an extremely thin layer of surfactant, which is currently assumed to play a noticeable role with regard to particle evacuation towards larger airway tubes. Particle clearance in the lung periphery is mainly characterized by the activity of alveolar macrophages, which clean the alveolar and bronchiolar surfaces from their particulate loads and transport the phagocytosed mass either towards the upper lung regions or towards the lung-associated lymph nodes. As an alternative clearance process, deposited particles again undergo a transepithelial evacuation. Based upon numerous experimental and theoretical studies (7-20), clearance of particles from the respiratory tract can be subdivided into three main phases with different length of time. Whilst fast bronchial clearance takes place within the first 24 h after particle exposure, slow bronchial and fast alveolar clearance exhibit clearance half-times of 5 to 25 d. Slow alveolar clearance, on the other hand, is commonly marked by a half-time of more than 100 d.
In the mathematical model used for the simulation of DEP clearance attribution of particulate mass to single clearance mechanisms depends upon three main parameters: (I) the deposition behavior of particles in various structures of the respiratory tract; (II) the size of the inhaled particulate substances; and (III) the shape of the particles. According to the theoretical approach small spherical particles have a higher probability to undergo slow bronchial clearance than large irregularly shaped particles (14,18-20). In the alveolar region extremely large (long) or extraordinarily small particles commonly escape from the uptake by macrophages and thus represent preferred targets of much slower clearance mechanisms (14,31). Mucus velocity in the upper and central airways is directly related to the bronchial and bronchiolar geometry of a stochastic lung model. As a consequence of this circumstance, transport velocities of particles captured on the mucociliary escalator also obtain a probabilistic behaviour. For slower clearance processes respective transfer velocities are randomly varied around their mean values. This is achieved by the random selection of velocity values from related probability density functions that have been already defined in previous contributions (14-20).
Model parameters
Theoretical clearance calculations were conducted for DEP aggregates varying in size between 50 and 250 nm. Deposition of these particles was produced after inhalation of the related aerosol under sitting breathing conditions with a tidal volume of 750 cm3 and a breathing frequency of 12 min−1 (breath-hold: 1s) (31). The stochastic lung with its numerous sequences of bronchial and bronchiolar airways was scaled for a functional residual capacity of 3,300 cm3, corresponding to the average lung size of a male caucasian adult. In general, clearance was expressed in terms of particle retention describing the ratio between retained and deposited particulate mass at a given point of time. Concretely speaking, 24-h, 10-d, and 100-d retentions were computed for all particle sizes in order to get a complete overview of fast and slow clearance processes.
Results
General features of DEP clearance from the respiratory tract
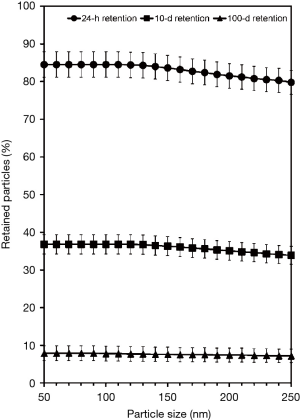
The effect of DEP size on the retention of particulate mass deposited in the respiratory tract is summarized in Figure 2. As can be clearly recognized from the related graph, the DEP fraction retained in the lungs after 24 h commonly ranges from 79.8% to 84.5%, whereby a negative correlation between particle size and retention values is predicted by the computer model. After 10 d the amount of DEP removed from the airways and alveoli varies between 33.9% and 36.8%. As an interesting feature, respective retention of 50-nm particles exceeds that of 250-nm particles by 2.9%, which indicates a remarkably slower clearance process of smaller aggregates. Retention values measured after 100 d and being mostly related to clearance processes in the most peripheral lung regions vary between 7.2% and 7.9%. Again, smaller DEP have a slightly higher potential to be permanently captured in the respective lung structures than large DEP.
Clearance courses of selected DEP
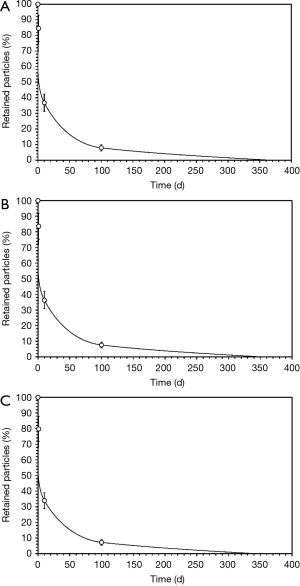
Based upon the retention values introduced in the preceding section, clearance curves have been computed for selected DEP (50, 150, 250 nm). Modeling results are summarized in Figure 3. Independent of the concerned particle size an exponential relationship between time on the one hand and particle retention on the other can be predicted. With proceeding duration of the clearance process the average slope of the clearance curve is subject to a continuous decrease. In a semi-logarithmic graph the sequence of single clearance phases described in the methods section is expressed by discontinuities (leap positions) in the respective functions. With the help of the clearance curves, however, an estimation of total clearance times can be carried out. According to the model predictions complete clearance of the selected DEP lasts from 324 to 365 d. Thereby, clearance time of 50-nm particles exceeds that of 250-nm particles by 11%.
Discussion
Since the middle of the last century it has been a well-known fact that the human lung disposes of several innate defense mechanisms, which are responsible for the clearance of inhaled particles deposited in various regions of the respiratory tract (1-10). In general, removal of particulate mass form the lung structures takes place via different transport routes (lung—gastrointestinal tract, lung—lymph nodes, lung—cardiovascular system) (14-20), along which particles are transferred with different velocities. Experimental studies could demonstrate that large particles (>1 µm) are mostly seized by fast clearance processes, so that more than 50% of the deposited substances are evacuated within a time span of 24 h (1-3). Small particles (<<1 µm), on the other hand, are increasingly subjected to slow clearance processes, which cause their retention in the lung structures for several weeks to months (5,6). In the case of increased penetration and deposition of particles in the alveoli, clearance commonly undergoes another retardation, with particulate fractions retaining in these structures for several years (34-39).
In the case of DEP aggregates varying in size between 50 and 250 nm, total clearance times ranging from 324 to 365 d could be predicted by the mathematical model. These values, however, have to be regarded as highly realistic and are confirmed by experimental research, where particles with similar physical characteristics were used (5-8). As another valuable result of the present contribution a negative correlation between particle size and retention times was computed. In other words, smaller DEP are retained in diverse lung structures for longer temporal intervals than larger DEP. This theoretical outcome can be evaluated as highly logical at first glance, because slow bronchial clearance fraction and particle size are characterized by a negative correlation, which was investigated in detail in preceding contributions (40-43). Additionally, smaller DEP deposited in the alveolar region increasingly undergo a transport on the surfactant as well as a transepithelial transfer, whereas larger DEP are preferential targets of alveolar macrophages. According to all these facts underlined by experimental and theoretical studies smaller particles bear a higher potential of a long-term residence in the lung compartments than larger particles (40-43).
An important question concerns the dependence of lung clearance on particle shape. Previous scientific investigation could furnish proof that particles with extreme anisometry (e.g., long fibers, carbon nanotubes with high aspect ratios) show a clearance behaviour, which significantly differs from that of spheres with volume-equivalent diameter (14,15,19,20). In the bronchial lung compartment anisometric particles commonly dispose of a higher probability to be captured on the mucus layer than spheres with identical volume. The latter particles may more easily pass mucus discontinuities and may subsequently reach the periciliary sol layer, where they are finally subjected to various slow clearance processes (14,15). In the alveolar compartment a completely different situation is given insofar as extremely anisometric particles with high biopersistence have the ability to remain in the lung bubbles for an undefined period of time. They neither can be engulfed permanently by alveolar macrophages nor can be endocytosed by epithelial cells (19-22). In the case of DEP, aggregate shape has only a minor effect on clearance efficiency due to several reasons: Firstly, particles originating from combustion of diesel fuels plot within a size frame, which predestinates them for slow bronchial clearance processes on the one hand and transepithelial alveolar clearance on the other hand (19-22). Secondly, DEP may become highly instable during their interaction with cellular substances, so that they partly fall apart into their components. This remarkable reduction in size, however, results in a further increase of the particulate mass removed via the transepithelial route.
Histological studies could demonstrate that DEP, which have been endocytosed by epithelial cells, are not automatically referred to a process of transcytosis, but can be also stored in the cytoplasm or cellular compartments for a non-determinable period of time (11-14). In this situation, however, the particles have to be regarded as serious health hazards, whereby highest injury to health emanates from diverse substances adsorbed on the DEP. Many of these inorganic and organic compounds had to be classified as carcinogens in the past, which have the potential to induce malignant transformations in the respective storage cells. Concerning this essential aspect, further experimental studies will have to be carried out in future.
From the results presented in this contribution it may be concluded that DEP deposited in the respiratory tract undergo very individual clearance processes. Duration of particle clearance depends on DEP size and the particle fractions accumulated in the bronchial airways and alveolar structures, respectively. The data provided by extensively validated clearance models may be, among other, used for the preparation of detailed risk assessments with regard to combustion particles.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2017.07.01). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Albert RE, Arnett LC. Clearance of radioactive dust from the human lung. AMA Arch Ind Health 1955;12:99-106. [PubMed]
- Albert RE, Lippmann M, Briscoe W. The characteristics of bronchial clearance in humans and the effects of cigarette smoking. Arch Environ Health 1969;18:738-55. [Crossref] [PubMed]
- Albert RE, Lippmann M, Peterson HT Jr, et al. Bronchial deposition and clearance of aerosols. Arch Intern Med 1973;131:115-27. [Crossref] [PubMed]
- Yu CP, Hu JP, Yen BM, et al. Models for mucociliary particle clearance in lung airways. In: Lee SD, Schneider T, Grant LD, et al. editors. Aerosols: Research, risk assessment and control strategies. Chelsea: MI, Lewis, 1986:569-78.
- Oberdörster G. Lung clearance of inhaled insoluble and soluble particles. J Aerosol Med 2009;1:289-330. [Crossref]
- Wolff RK. Mucociliary Function. In: Parent RA. editor. Comparative Biology of the Normal Lung. New York: CRC Press, 1989:659-80.
- Stahlhofen W, Gebhart J, Rudolf G, et al. Measurement of lung clearance with pulses of radioactivity-labelled aerosols. J Aerosol Sci 1986;17:330-6. [Crossref]
- Stahlhofen W, Koebrich R, Rudolf G, et al. Short-term and long-term clearance of particles from the upper human respiratory tract as a function of particle size. J Aerosol Sci 1990;21:S407-10. [Crossref]
- Scheuch G, Stahlhofen W, Heyder J. An approach to deposition and clearance measurements in human airways. J Aerosol Med 1996;9:35-41. [Crossref] [PubMed]
- Lippmann M, Yeates DB, Albert RE. Deposition, retention, and clearance of inhaled particles. Br J Ind Med 1980;37:337-62. [PubMed]
- Sturm R, Hofmann W. Mechanistic interpretation of the slow bronchial clearance phase. Radiat Prot Dosimetry 2003;105:101-4. [Crossref] [PubMed]
- Hofmann W, Sturm R. Stochastic model of particle clearance in human bronchial airways. J Aerosol Med 2004;17:73-89. [Crossref] [PubMed]
- Sturm R, Hofmann W. Stochastic modeling predictions for the clearance of insoluble particles from the tracheobronchial tree of the human lung. Bull Math Biol 2007;69:395-415. [Crossref] [PubMed]
- Sturm R, Hofmann W. A theoretical approach to the deposition and clearance of fibers with variable size in the human respiratory tract. J Hazard Mater 2009;170:210-8. [Crossref] [PubMed]
- Sturm R. A computer model for the simulation of fiber-cell interaction in the alveolar region of the respiratory tract. Comput Biol Med 2011;41:565-73. [Crossref] [PubMed]
- Sturm R, Hofmann W, Scheuch G, et al. Particle clearance in human bronchial airways: comparison of stochastic model predictions with experimental data. Ann Occup Hyg 2002;46:329-33. [PubMed]
- Sturm R, Hofmann W. A multi-compartment model for slow bronchial clearance of insoluble particles--extension of the ICRP human respiratory tract models. Radiat Prot Dosimetry 2006;118:384-94. [Crossref] [PubMed]
- Sturm R. Deposition and cellular interaction of cancer-inducing particles in the human respiratory tract: Theoretical approaches and experimental data. Thoracic Cancer 2010;4:141-52. [Crossref]
- Sturm R. Theoretical and experimental approaches to the deposition and clearance of ultrafine carcinogens in the human respiratory tract. Thorac Cancer 2011;2:61-8. [Crossref]
- Sturm R. Theoretical models of carcinogenic particle deposition and clearance in children’s lungs. J Thorac Dis 2012;4:368-76. [PubMed]
- Sturm R. Theoretical models for dynamic shape factors and lung deposition of small particle aggregates originating from combustion processes. Z Med Phys 2010;20:226-34. [Crossref] [PubMed]
- Hofmann W, Sturm R, Winkler-Heil R, et al. Stochastic model of ultrafine particle deposition and clearance in the human respiratory tract. Radiat Prot Dosimetry 2003;105:77-80. [Crossref] [PubMed]
- Butterweck G, Vezzù G, Schuler C, et al. In vivo measurement of unattached radon progeny deposited in the human respiratory tract. Radiat Prot Dosimetry 2001;94:247-50. [Crossref] [PubMed]
- Geiser M, Rothen-Rutishauser B, Knapp N, et al. Ultrafine particles cross cellular membranes by nonphagocytotic mechanisms in lungs and in cultured cells. Environ Health Perspect 2005;113:1555-60. [Crossref] [PubMed]
- Asgharian B, Hofmann W, Miller FJ. Mucociliary clearance of insoluble particles from the tracheobronchial airways of the human lung. J Aerosol Sci 2001;32:817-32. [Crossref]
- Lee PS, Gerrity TR, Hass FJ, et al. A model for tracheobronchial clearance of inhaled particles in man and a comparison with data. IEEE Trans Biomed Eng 1979;26:624-30. [Crossref] [PubMed]
- Gradoń L, Podgórski A. Kinetics of particle retention in the human respiratory tract. Ann Occup Hyg 1991;35:249-59. [PubMed]
- Sturm R. A computer model for the clearance of insoluble particles from the tracheobronchial tree of the human lung. Comput Biol Med 2007;37:680-90. [Crossref] [PubMed]
- Sturm R. Age-dependence and intersubject variability of tracheobronchial particle clearance. Pneumon 2011;24:77-84.
- Sturm R. An advanced stochastic model for mucociliary particle clearance in cystic fibrosis lungs. J Thorac Dis 2012;4:48-57. [PubMed]
- International Commission on Radiological Protection (ICRP). Human Respiratory Tract Model for Radiological Protection. Oxford: Pergamon Press, 1994.
- Sturm R. Bioaerosols in the lungs of subjects with different ages-part 1: deposition modeling. Ann Transl Med 2016;4:211. [Crossref] [PubMed]
- Sturm R. Clearance of carbon nanotubes in the human respiratory tract-a theoretical approach. Ann Transl Med 2014;2:46. [PubMed]
- Lippmann M. Effects of fiber characteristics on lung deposition, retention, and disease. Environ Health Perspect 1990;88:311-7. [Crossref] [PubMed]
- Sturm R. Theoretical approach to the hit probability of lung-cancer-sensitive epithelial cells by mineral fibers with various aspect ratios. Thoracic Cancer 2010;3:116-25. [Crossref]
- Sturm R. A three-dimensional model of tracheobronchial particle distribution during mucociliary clearance in the human respiratory tract. Z Med Phys 2013;23:111-9. [Crossref] [PubMed]
- Sturm R. Theoretical models for the simulation of particle deposition and tracheobronchial clearance in lungs of patients with chronic bronchitis. Ann Transl Med 2013;1:3. [PubMed]
- Sturm R. Modeling the delay of mucous flow at the carinal ridges of the human tracheobronchial tree. Comp Math Biol 2014;3:6.
- Sturm R. An advanced mathematical model of slow bronchial clearance in the human respiratory tract. Comp Math Biol 2016;5:2.
- Sturm R. Modeling the deposition of bioaerosols with variable size and shape in the human respiratory tract - A review. J Adv Res 2012;3:295-304. [Crossref]
- Sturm R, Hofmann W. A computer program for the simulation of fiber deposition in the human respiratory tract. Comput Biol Med 2006;36:1252-67. [Crossref] [PubMed]
- Sturm R. Nanotubes in the respiratory tract - Deposition modeling. Z Med Phys 2015;25:135-45. [Crossref] [PubMed]
- Sturm R. A stochastic model of carbon nanotube deposition in the airways and alveoli of the human respiratory tract. Inhal Toxicol 2016;28:49-60. [Crossref] [PubMed]
Cite this article as: Sturm R. Theoretical simulation of diesel exhaust particle clearance from the human respiratory tract. J Public Health Emerg 2017;1:74.


