
Aerosol bolus dispersion in male and female subjects: a theoretical analysis
Introduction
General aspects
In the past decades, aerosol bolus dispersion produced by specific inhalation experiments has attracted enhanced physical and medical attention due to its potential as diagnostic technique for the detection of various lung diseases (1-10). In the healthy lung, an inspired aerosol pulse containing a predefined concentration of sub-micron particles is subject to a continuous widening during its transport through the air-conducting structures of the respiratory tract. This phenomenon is largely explicable by the processes of convective mixing taking place in the bronchial tubes and alveolar air sacs. Both experimental and theoretical studies could demonstrate that the mixing procedure is controlled by a multitude of physical and physiological factors, among which the flow rate of the inhaled air, the inspired air volume, particle properties, and lung morphometry have highest relevance (11-15). Those lung diseases inducing a permanent reduction of the airway calibres [e.g., asthma, chronic bronchitis, chronic obstructive pulmonary disease (COPD)] are commonly reflected by decreased dispersion of the inhaled aerosol bolus, whereas insufficiencies causing a remarkable and non-reversible change of the peripheral lung architecture (e.g., emphysema, bronchiectasis) are expressed by a valuable increase of aerosol bolus dispersion (2-5).
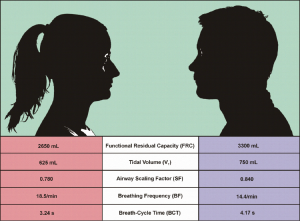
As comprehensively discussed in earlier publications (16-18), average lung morphometry determined for male subjects measurably differs from that evaluated for female subjects. As a direct consequence of this morphometric discrepancy, females inhale smaller air volumes than males and thus need more breath-cycles per minute in order to supply their organs with sufficient oxygen (Figure 1). In addition, particle deposition studies furnished proof that smaller female lungs often represent a more preferential target for the accumulation of particulate mass than larger male lungs (19-23). Although lung medicine has made increased efforts with regard to the working out of gender-related breathing behaviour, lots of questions affecting this problem are still unacknowledged.
One of these questions, which has not been answered in detail yet, concerns possible effects of gender-specific lung morphometry on aerosol bolus dispersion. Previous experimental studies using bolus inhalation recruited both female and male probands (1-6,21-23). Results obtained from the inhalation tests were subsequently averaged over all subjects participating in the investigation, so that potential gender-related differences could not be reproduced appropriately. The comprehension of such discrepancies, however, represents a basic prerequisite for a successful establishment of the aerosol bolus technique as diagnostic tool.
Main objectives of the present study
The present contribution is primarily thought (I) to work out the main contrasts between male and female aerosol bolus inhalation and (II) to provide a profound theoretical basis for future experiments adding gender-specificities to their frame conditions. The study hypothesizes that statistical parameters describing aerosol bolus behaviour in the structures of the respiratory system partly show significant differences between males and females due to the circumstances noted above. Also particle exit from the bolus and subsequent deposition on the bronchial, bronchiolar, and alveolar walls are assumed to take place in a gender-related fashion.
Methods
Description of the aerosol bolus dispersion model
Since the aerosol bolus dispersion model has been already subjected to a comprehensive description in numerous previous studies (24-30), only its most important cornerstones will be outlined here. Form a physical point of view, widening of the aerosol bolus during its transport through the respiratory structures has to be regarded as result of two phenomena: (I) convective mixing in the air-conducting bronchi and bronchioli and (II) the mixture of a certain fraction of tidal air (and the particles transported therein) with residual air stored in the alveoli. Consideration of these physical processes took place by application of both empirical and numerical equations (27-30). Mathematical expression of convective mixing was conducted by definition of effective diffusivities for inhalation and exhalation of the particle peak. In general, effective diffusivities positively correlate with transport velocity of the inhaled air through the bronchial system and the radial size of the airway structures, but show a negative correlation with the size of particles transported in the boluses. As effective diffusivities represent mean values being valid for the whole airway, they proved to be inappropriate for the computation of mixing effects occurring at certain points of the inhalation and exhalation path (27-30). In order to overcome this disadvantage, the time of an aerosol particle to pass a bronchial tube of given length was randomly selected from a related time-distribution function (27).
In the alveolar region, the behaviour of aerosol bolus particles is founded upon the circumstance that besides an ideal mixing between tidal and residual air also a non-mixing between these two media has to be theoretically considered and included into the approach. This non-mixing is simulated with the help of the ‘first-in-last-out’ statement, according to which particles, which have entered an alveolus at the very beginning of the inhalation period, leave that alveolus again at the very end of the inhalation period. The mathematical relation between ideally mixed air and tidal volume is currently expressed in the model by an empirical mixing factor, which is committed to the value 0.25 (i.e., 25% ideal mixing and 75% ‘first-in-last-out’ approximation) (7-9,24-26).
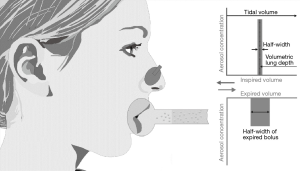
For a precise mathematical characterization of modifications of aerosol bolus shape during intrapulmonary transport, several descriptive parameters have been computed (Table 1). Dispersion of the particle peak is best expressed by a theoretical measurement of the half-width of the exhaled bolus, for which a Newtonian approach finds its application. Further parameters for a description of width and shape of the exhaled aerosol bolus are evaluated by analysis of normalized statistical moments (7-9,24,27). The standard deviation of the exhaled aerosol bolus is simply derived from the second statistical moment, whereas the skewness of the exhaled aerosol bolus may be derived from the third statistical moment. Position of the aerosol bolus within the exhaled air stream is determined by a numerical estimation of the peak mode of the expired aerosol bolus and its site-related comparison with the mode of the inspired bolus (Figure 2; Table 1).
Table 1
| Aerosol bolus parameter | Mathematical approach | Sketch |
|---|---|---|
| Half-width (HW) of the exhaled aerosol bolus | Newtonian approach | |
| Standard deviation (σ) of the exhaled aerosol bolus | ||
| Skewness (ψ) of the exhaled aerosol bolus | ||
| Mode-shift (ΔM) | ΔM = VLD − M |
C(t) = particle concentration at time t; tI = time of bolus insertion into the inhaled air; tm = time of bolus median.
Simulation of particle deposition
Deposition of particles transported in the aerosol bolus was simulated by application of both empirical and analytical equations describing the effect of different physical mechanisms (Brownian motion, inertial impaction, sedimentation) on the particulate bodies (16,31). As already outlined in previous publications (32-50), all deposition mechanisms particularly exhibit a dependence on particle size and velocity of the inhaled air stream. Therefore, inertial impaction mainly affects the upper airways of the respiratory tract, whereas sedimentation has its highest efficiency in the peripheral airway structures and alveolar spheres. In the case of slow inhalation and very small particle dimensions, Brownian motion may be regarded as highly effective in the large bronchial air passages, whilst fast inspiration commonly results in a successive displacement of diffusion-induced deposition towards central and peripheral airways and alveoli. In order to obtain higher accuracy of deposition predictions for the upper airway generations, effects of the laryngeal jet in the trachea and secondary air flows at the bifurcations were taken into account by means of correction factors (31-35).
Model parameters used in this study
Generation of modeling predictions took place by assumption of a constant breath-cycle time of 8 s. Additionally, symmetric inhalation and exhalation and the absence of an intercalated breath-hold were considered. Tidal volume was uniformly set to 1,000 mL, which resulted in an inspiration flow rate of 250 mL/s. Half-width of the inhaled aerosol bolus was committed to 50 mL and its insertion of the aerosol peak into the inspired air took place after 100 mL (0.4 s), 200 mL (0.8 s), 300 mL (1.2 s), 400 mL (1.6 s), 500 mL (2.0 s), 600 mL (2.4 s), 700 mL (2.8 s), and 800 mL (3.2 s). Inhaled boluses were injected with monodisperse spherical particles with a diameter of 0.84 µm (24) and unit-density (1 g/cm3).
Results
Parameters describing aerosol bolus behaviour in male and female lungs
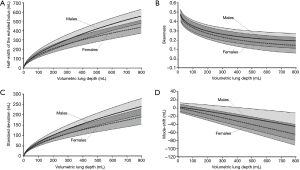
Results obtained from the modeling predictions are summarized in Figures 3 and 4. In Figure 3 all statistical parameters related to aerosol bolus inhalation are expressed as functions of the volumetric lung depth (VLD; Figure 2). This physiological quantity denotes the air volume, which is measured from the end of inhalation to the insertion of the aerosol pulse. Low values of the VLD mark a position of the bolus at late inhalation stages (shallow bolus inhalation), whereas high values of the VLD correspond to early aerosol bolus inspiration (7-9,24). The basic principle behind the relationship between aerosol bolus parameters and VLD is the generation of a simple method of controlled particle transport to predefined target regions of the lungs, thereby reflecting the value of the aerosol bolus technique for inhalation therapy. Starting with the half-width of the exhaled aerosol bolus, both male and female probands develop an exponential dependence of this parameter on the VLD. In male subjects half-width is subject to an increase from 0 mL (VLD =0 mL) to 552±74 mL (mean ± CI-99%; VLD =800 mL), whereas in females the same parameter rises from 0 mL (VLD =0 mL) to 435±69 mL (VLD =800 mL; Figure 3A). As depicted in Figure 4A, where female half-width values are plotted against male ones, gender-related differences of this statistical bolus parameter commonly range from 7% to 40% and have to be evaluated as highly significant (P<0.01) over the observed range of VLD. The standard deviation of the aerosol bolus transported through the different structures of the respiratory tract also follows an exponential function and assumes values between 0 mL (VLD =0 mL) and 242±38 mL (VLD =800 mL) in male probands. In female subjects, on the other hand, the respective function is characterized by an increase from 0 mL (VLD =0 mL) to 183±41 mL (VLD =800 mL; Figure 3B). Differences of this statistical parameter between males and females are on the order of 0 to 47% (Figure 4B) and thus reach partly remarkable dimensions. Skewness of the aerosol bolus, representing a reliable measure of peak asymmetry, commonly develops from 0.53±0.05 (VLD =0 mL) to 0.22±0.08 (VLD =800 mL) in male subjects, but from 0.50±0.04 (VLD =0 mL) to 0.15±0.10 (VLD =800 mL) in female probands (Figure 3C). As exhibited in the related graph, dependence of this parameter on the VLD is also best expressed by an exponential function. In this case, differences between males and females range from 0 to 78% (Figure 4C). The mode-shift, expressing a slight displacement of highest aerosol concentration during intrapulmonary peak transport, may be generally described by negatively sloped linear functions, which adopt values between 2±11 mL (VLD =0 mL) and −45±33 mL (VLD =800 mL) in males and between 0±10 mL (VLD =0 mL) and −66±25 mL (VLD =800 mL) in females (Figure 3D). Here, gender-related differences depicted in Figure 4D vary between 0 to 82%.
Gender-related differences of particle deposition from the aerosol bolus
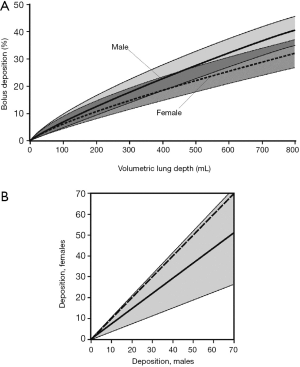
Besides the statistical parameters related to aerosol bolus inhalation also deposition of particles transported in this aerosol peak is subject to a gender-specific behaviour (Figure 5). In general, dependence of particle deposition on the VLD may be again described by exponential functions, reflecting a continuous increase of intrapulmonary particle accumulation from shallow to deep bolus inhalation. In male probands deposition develops from 0.0% (VLD =0 mL) to 39.1%±6.2% (VLD =800 mL), whereas in female subjects an enhancement of this parameter from 0.0% (VLD =0 mL) to 30.8%±5.9% (VLD =800 mL) may be recognized (Figure 5A). Discrepancies of particle deposition between males and females are summarized in Figure 5B and range from 0 to 60%. Over the whole range of VLDs they are characterized by high significance (P<0.01).
Discussion and conclusions
Early experimental and epidemiological studies dealing with inhalation and intrapulmonary deposition of hazardous particles were primarily focused on male probands (16), because particle behaviour in female lungs was assumed to be almost identical to that in male lungs. Comprehensive morphometric studies starting in the 1960s (31) could unveil remarkable differences of average lung size between adult men and women. Later on, it was also found by numerous experiments that females exhibit a breathing habit differing remarkably from the male one (Figure 1) (16). Even clearance of particulate mass from the respiratory tract was evaluated as gender-specific process (16,18), because mucus velocities in the bronchi and bronchioli were found to depend on the size of the tubular structures. Since the beginning of this century, enhanced efforts have been undertaken in order to work out gender-related specificities of particle inhalation and deposition and to develop respective risk assessments separated by gender.
In the past decades, aerosol bolus inhalation was used for the clarification of numerous medical problems and additionally was developed as highly promising technique of non-invasive diagnosis of various lung diseases (1-10). Although most experimental studies included both male and female probands, possible discrepancies of bolus behaviour between the genders did not represent the center points of the investigations. In most cases it was argued that selection of the test subjects took place according to a specific protocol, where physiological parameters only varied within fixed limits. In other words, only probands showing a respiratory physiology matching the experimental requirements were admitted to the inhalation tests. The present contribution, however, yields evidence that transport and physical behaviour of aerosol boluses are characterized by certain gender-related peculiarities.
If the inhalation procedure (tidal volume, flow rate, inhalation time) is subject to a standardization among male and female probands, theoretical computations suggest a stronger accentuation of aerosol bolus dispersion and peak deformation in the lungs of men. In concrete figure, gender-related differences of statistical parameters describing bolus development in the bronchial and alveolar structures are on the order of 0 to 80%. For deep bolus inspiration (VLD >700 mL) discrepancies of some parameters (half-width, standard deviation) have to be classified as highly significant (P<0.01) and therefore have to be considered with regard to the creation of aerosol-bolus-based procedures of disease diagnosis and inhalation therapies. In this context it has to be mentioned additionally that the lungs of male and female children and adolescents (5–15 y) are not marked by similar morphometric differences as those of adults, for what reason the presented results are strictly limited to the adult age group (20-23). Another problem concerns the normal breathing behaviour of men and women being observable in everyday life. Although males commonly perform an inspiration of atmospheric aerosols into deeper parts of the lungs, resulting in higher alveolar particle deposition, females usually show a higher breathing frequency and thus achieve a similar burden of the alveolar structures with particulate mass within a given time period (16,19-22).
Main reasons of the gender-related aerosol bolus behaviour predicted in this contribution are quickly detected. Under the assumption of standardized breathing conditions smaller female lungs take up the identical air volume (e.g., 1,000 mL) within a given time interval as larger male lungs do. This results in higher velocities of the air stream in the upper airways of female probands, but also a much lower residence time of particulate mass in single lung generations. As consequence of these two antagonistic phenomena a less accentuated axial dispersion of the aerosol transported in the bolus may be observed. Increased particle deposition in males is also easily explicable by the gender-specific differences of bronchial morphometry, because in female airways lower radial distances are concealed by higher transport velocities and faster airway passages of the inhaled particles. This results in an enhancement of probabilities of hitting the bronchial and bronchiolar walls in male lungs (31-50).
From the results presented in this study it may be concluded that aerosol bolus inhalation taking place under standard conditions is marked by non-negligible gender-specificities, which have to be subjected to detailed experimental investigations in the near future. Only detailed knowledge of gender-related aerosol bolus behaviour entitles lung medicine to use this inhalation technique in clinical diagnosis.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2017.04.01). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anderson PJ, Hardy KG, Gann LP, et al. Detection of small airway dysfunction in asymptomatic smokers using aerosol bolus behavior. Am J Respir Crit Care Med 1994;150:995-1001. [Crossref] [PubMed]
- Anderson PJ, Blanchard JD, Brain JD, et al. Effect of cystic fibrosis on inhaled aerosol boluses. Am Rev Resp Dis 1989;140:1317-24. [Crossref] [PubMed]
- Schulz H, Schulz A, Brand P, et al. Aerosol bolus dispersion and effective airway diameters in mildly asthmatic children. Eur Respir J 1995;8:566-73. [PubMed]
- Verbanck S, Schuermans D, Vincken W, et al. Saline aerosol bolus dispersion. I. The effect of acinar airway alteration. J Appl Physiol 2001;90:1754-62. [PubMed]
- Kohlhäufl M, Brand P, Rock C, et al. Noninvasive Diagnosis of Emphysema. Am J Resp Crit Care Med 1999;160:913-8. [Crossref] [PubMed]
- McCawley M, Lippmann M. Development of an aerosol dispersion test to detect early changes in lung function. Am Ind Hyg Assoc J 1988;49:357-66. [Crossref] [PubMed]
- Sturm R, Pawlak E, Hofmann W. Monte-Carlo-Modell der Aerosolbolusdispersion in der menschlichen Lunge – Teil 1: Theoretische Modellbeschreibung und Anwendung. Z med Phys 2007;17:127-35. [Crossref] [PubMed]
- Sturm R, Pawlak E, Hofmann W. Monte-Carlo-Modell der Aerosolbolusdispersion in der menschlichen Lunge – Teil 2: Modellvorhersagen für die kranke Lunge. Z med Phys 2007;17:136-43. [Crossref] [PubMed]
- Sturm R. Aerosol bolus dispersion in healthy and asthmatic children-theoretical and experimental results. Ann Transl Med 2014;2:47. [PubMed]
- Sturm R. Aerosol bolus inhalation as technique for the diagnosis of various lung diseases – a theoretical approach. Comp Math Biol 2014;3:2.
- Brown JS, Gerrity TR, Bennett WD, et al. Dispersion of aerosol boluses in the human lung: dependence on lung volume, bolus volume, and gender. J Appl Physiol (1985) 1995;79:1787-95. [PubMed]
- Rosenthal FS, Blanchard JD, Anderson PJ. Aerosol bolus dispersion and convective mixing in human and dog lungs and physical models. J Appl Physiol (1985) 1992;73:862-73. [PubMed]
- Blanchard JD. Aerosol bolus dispersion and aerosol-derived airway morphometry: assessment of lung pathology and response to therapy, Part 1. J Aerosol Med 1996;9:183-205. [Crossref] [PubMed]
- Schulz H, Eder G, Heyder J. Lung volume is a determinant of aerosol bolus dispersion. J Aerosol Med 2003;16:255-62. [Crossref] [PubMed]
- Schulz H, Heilmann P, Hillebrecht A, et al. Convective and diffusive gas transport in canine intrapulmonary airways. J Appl Physiol 1992;72:1557-62. [PubMed]
- International Commission on Radiological Protection (ICRP). Human Respiratory Tract Model for Radiological Protection, Publication 66. Oxford: Pergamon Press, 1994.
- Sturm R. Modeling the deposition of bioaerosols with variable size and shape in the human respiratory tract - A review. J Adv Res 2012;3:295-304. [Crossref]
- Sturm R. Age-dependence and intersubject variability of tracheobronchial particle clearance. Pneumon 2011;24:77-85.
- Sturm R. Local lung deposition of ultrafine particles in healthy adults: experimental results and theoretical predictions. Ann Transl Med 2016;4:420. [PubMed]
- Sturm R. Total deposition of ultrafine particles in the lungs of healthy men and women: experimental and theoretical results. Ann Transl Med 2016;4:234. [Crossref] [PubMed]
- Bennett WD, Zeman KL, Kim C. Variability of fine particle deposition in healthy adults: effect of age and gender. Am J Respir Crit Care Med 1996;153:1641-7. [Crossref] [PubMed]
- Jaques PA, Kim CS. Measurement of total lung deposition of inhaled ultrafine particles in healthy men and women. Inhal Toxicol 2000;12:715-31. [Crossref] [PubMed]
- Kim CS, Jaques PA. Analysis of total respiratory deposition of inhaled ultrafine particles in adult subjects at various breathing patterns. Aerosol Sci Technol 2004;38:525-40. [Crossref]
- Brand P, Rieger C, Schulz H, et al. Aerosol bolus dispersion in healthy subjects. Eur Respir J 1997;10:460-7. [Crossref] [PubMed]
- Hofmann W, Pawlak E, Sturm R. Semi-empirical stochastic model of aerosol bolus dispersion in the human lung. Inhal Toxicol 2008;20:1059-73. [Crossref] [PubMed]
- Sturm R. Aerosol bolus inhalation in subjects with different age – a theoretical approach. Comp Math Biol 2014;3:7.
- Scherer PW, Shendalman LH, Greene NM, et al. Measurement of axial diffusivities in a model of the bronchial airways. J Appl Physiol 1975;38:719-23. [PubMed]
- Sarangapani R, Wexler AS. Modeling aerosol bolus dispersion in human airways. J Aerosol Sci 1999;30:1345-62. [Crossref]
- Lee JW, Lee DY, Kim WS. Dispersion of an aerosol bolus in a double bifurcation. J Aerosol Sci 2000;31:491-505. [Crossref]
- Darquenne C, Brand P, Heyder J, et al. Aerosol dispersion in human lung: comparison between numerical simulations and experiments for bolus tests. J Appl Physiol (1985) 1997;83:966-74. [PubMed]
- Koblinger L, Hofmann W. Monte Carlo modeling of aerosol deposition in human lungs. PartI: Simulation of particle transport in a stochastic lungstructure. J Aerosol Sci 1990;21:661-74. [Crossref]
- Sturm R, Hofmann W. Stochastic simulation of alveolar particle deposition in lungs affected by different types of emphysema. J Aerosol Med 2004;17:357-72. [Crossref] [PubMed]
- Sturm R. Bioaerosols in the lungs of subjects with different ages - part 1: deposition modeling. Ann Transl Med 2016;4:211. [Crossref] [PubMed]
- S Sturm R.. A computer model for the simulation of fiber-cell interaction in the alveolar region of the respiratory tract. Comput Biol Med 2011;41:565-73. [Crossref] [PubMed]
- Sturm R. Theoretical models of carcinogenic particle deposition and clearance in children’s lungs. J Thorac Dis 2012;4:368-76. [PubMed]
- Sturm R. Deposition and cellular interaction of cancer-inducing particles in the human respiratory tract: Theoretical approaches and experimental data. Thoracic Cancer 2010;4:141-52. [Crossref]
- Sturm R, Hofmann W. A theoretical approach to the deposition and clearance of fibers with variable size in the human respiratory tract. J Hazard Mater 2009;170:210-8. [Crossref] [PubMed]
- Sturm R. Theoretical approach to the hit probability of lung-cancer-sensitive epithelial cells by mineral fibers with various aspect ratios. Thoracic Cancer 2010;3:116-25. [Crossref]
- Sturm R. Theoretical and experimental approaches to the deposition and clearance of ultrafine carcinogens in the human respiratory tract. Thoracic Cancer 2011;2:61-8. [Crossref]
- Sturm R. Theoretical models for the simulation of particle deposition and tracheobronchial clearance in lungs of patients with chronic bronchitis. Ann Transl Med 2013;1:3. [PubMed]
- Sturm R, Hofmann W. A computer program for the simulation of fiber deposition in the human respiratory tract. Comput Biol Med 2006;36:1252-67. [Crossref] [PubMed]
- Sturm R. Theoretical models for dynamic shape factors and lung deposition of small particle aggregates originating from combustion processes. Z med Phys 2010;20:226-34. [Crossref] [PubMed]
- Sturm R. Nanotubes in the respiratory tract - Deposition modeling. Z med Phys 2015;25:135-45. [Crossref] [PubMed]
- Sturm R. A stochastic model of carbon nanotube deposition in the airways and alveoli of the human respiratory tract. Inhal Toxicol 2016;28:49-60. [Crossref] [PubMed]
- Sturm R. Spatial visualization of nanoparticle deposition in the human respiratory tract. Ann Transl Med 2015;3:326. [PubMed]
- Sturm R. Inhaled nanoparticles. Phys Today 2016;69:70-1. [Crossref]
- Sturm R. Theoretical deposition of nanotubes in the respiratory tract of children and adults. Ann Transl Med 2014;2:6. [PubMed]
- Sturm R, Hofmann W 3D. -Visualization of particle deposition patterns in the human lung generated by Monte Carlo modeling: methodology and applications. Comput Biol Med 2005;35:41-56. [Crossref] [PubMed]
- Sturm R. A computer model for the simulation of nonspherical particle dynamics in the human respiratory tract. Phys Res Intern 2012:1-11.
- Sturm R. Theoretical deposition of carcinogenic particle aggregates in the upper respiratory tract. Ann Transl Med 2013;1:25. [PubMed]
Cite this article as: Sturm R. Aerosol bolus dispersion in male and female subjects: a theoretical analysis. J Public Health Emerg 2017;1:46.


