
Association between endocrine function and radiation exposure
Introduction
There has been a growing focus on endocrine dysfunction especially thyroid dysfunction after radiation exposure recent years, which may impact on morbidity even mortality. Thyroid dysfunction contains hyperthyroidism, hypothyroidism and some subclinical thyroid dysfunction, which may be associated with dyslipidemia (1-3), which has been proved to be a risk factor for ischemic heart disease and the all-cause mortality (4,5). With respect to this side, the relation between changes in thyroid function and radiation exposure might be important, as radiation is associated with increased thyroid dysfunction as well as morbidity (6-9). There have been many epidemiological studies placing emphasis on thyroid function (10-13), but little has paid attention to the relation between radiation and thyroid function.
Thyroid dysfunction caused by radiation exposure or other reasons may be associated with change in body mass index (BMI), weight and even induced obesity and metabolic consequences including diabetes (14-16). On the basis of above theories, we decided to observe the likely relationship of endocrine function changes (such as thyroid function and glucose metabolism) within radiation exposure. In the current study, we explored glucose metabolism and thyroid function parameters. We have gathered data from the physical examination center, which has general health survey arranged for occupational health. Most of radiation workers participated were exposed to X-ray, which gave us the opportunity to research only one type of radial without interference and to evaluate the relation between some hormone levels about endocrine system and radiation exposure in a cross-sectional stage.
Methods
Subjects and questionnaire
The study enrolled hospital radiation operators most of whom were radiologists, they were recruited consecutively at the physical examination organization for occupational health from year 2015 to 2016. The annual effective dose equivalents of all subjects were less than 0.24 mSv, which was far below our national standard (10 mSv).
Subject information was collected with a questionnaire that was carried out through interviews in the forms of face-to-face. The questionnaire generally included demographic data, medical conditions including previous and present, radiation exposure, pharmaceutical preparations, previous radiation exposure to X-ray, hereditary factors, smoking and drinking status. In our study, ever drinkers was identified as subjects who drank a bottle of beer or fifty grams of wine per day and for at least one year, however, the rest were never ones. Workers who had one cigarette per day for at least one year were identified as ever ones, all others were never smokers.
Blood glucose and thyroid related hormones
Overnight fasting plasma glycemia were detected from blood samples that were collected in the morning. Serum levels of FT3 (free triiodothyronine), T3 (triiodothyronine), TSH (thyroid stimulating hormone), T4 (thyroxine) and FT4 (free thyroxine) were determined using a commercially available ELISA kit according to the specifications. A glucometer was used to detect serum glucose.
Statistical analysis
We entered all data into a computerized database using the statistical analysis Epidata 3.1, all analyses were performed by SAS 9.1.3 (SAS Institute, Cary, NC, USA) and SPSS 22.0 (SPSS Inc., USA) software. Student’s t-tests and Univariate analysis of variance were used for the analysis of continuous data. Qualitative data were evaluated using Pearson χ2 test. Relationships between BMI, plasma glucose and thyroid hormones were analyzed with spearman’s correlation analysis. P<0.05 was generally accepted as statistically significant.
Results
In all, 2,129 subjects participated in this survey. All subjects exposed to other types of radiation except for X-ray (n=192) were excluded. Of the 1,937 remaining subjects, 153 were excluded because of missing values for one or more analyzed factors, finally leaving 1,784 subjects to be included in the analyses.
Descriptive characteristics of all subjects including many factors with significant association are shown in Table 1. The abnormal proportion of T3 and T4 in female were higher than them in male, with rate 1.3% of T3 and 1.5% of T4 in female compared to 0.2% and 0.4% in male (P<0.05), respectively. Abnormal rate of FT4 increased, accompanying with the growth of age (P<0.05). Nevertheless, abnormal rate of T4 decreased with BMI rose (P<0.05). There is a little incredible to detect that smokers were unlikely to approach abnormalities of T3 and TSH, on the contrary, subjects who never smoke may be susceptible to abnormality potentially (P<0.05), with frequency 0.0% of T3 and 3.3% of TSH in non-smokers, compared to 0.75% of T3 and 5.5% of TSH in ever smokers. We were pleased to observe the relation between the degree of education and FT4 level. In the group of junior high school, abnormal proportion of FT4 was 4.2%, while in group high school degree, it decreased to 1.0% and further to 0.6% in college and above obviously (P<0.05). In addition, T3 and FT3 levels may be associated with exposure time of X-ray. On the basis of this survey, abnormal rate of T3 (1.4%) and FT3 (1.0%) were higher in group <3 years than that in group ≥3 years (0.3% and 0.1%, respectively; P<0.05). However, there may be no significant association between thyroid-related hormone level and drinking status, safeguard procedures.
Table 1
| Variable | Total | T3 | T4 | TSH | FT3 | FT4 | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | P | Normal | Abnormal | P | Normal | Abnormal | P | Normal | Abnormal | P | Normal | Abnormal | P | |||||||||||||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||||||||||||||||
| Sex | |||||||||||||||||||||||||||||||||||||
| Male | 1,250 | 100.0 | 1,248 | 99.8 | 2 | 0.2 | 1,245 | 99.6 | 5 | 0.4 | 1,196 | 95.7 | 54 | 4.3 | 1,248 | 99.8 | 2 | 0.2 | 1,240 | 99.2 | 10 | 0.8 | |||||||||||||||
| Female | 534 | 100.0 | 527 | 98.7 | 7 | 1.3 | 0.004 | 526 | 98.5 | 8 | 1.5 | 0.018 | 502 | 94.0 | 32 | 6.0 | 0.084 | 531 | 99.4 | 3 | 0.6 | 0.162 | 530 | 99.3 | 4 | 0.7 | 0.587 | ||||||||||
| Age (years) | |||||||||||||||||||||||||||||||||||||
| <30 | 588 | 100.0 | 583 | 99.1 | 5 | 0.9 | 585 | 99.5 | 3 | 0.5 | 560 | 95.2 | 28 | 4.8 | 587 | 99.8 | 1 | 0.2 | 585 | 99.5 | 3 | 0.5 | |||||||||||||||
| 30–41 | 595 | 100.0 | 592 | 99.5 | 3 | 0.5 | 591 | 99.3 | 4 | 0.7 | 572 | 96.1 | 23 | 3.9 | 591 | 99.3 | 4 | 0.7 | 593 | 99.7 | 2 | 0.3 | |||||||||||||||
| >41 | 600 | 100.0 | 599 | 99.8 | 1 | 0.2 | 0.251 | 594 | 99.0 | 6 | 1.0 | 0.599 | 565 | 94.2 | 35 | 5.8 | 0.283 | 600 | 100.0 | 0 | 0.0 | 0.074 | 591 | 98.5 | 9 | 1.5 | 0.049 | ||||||||||
| Smoke | |||||||||||||||||||||||||||||||||||||
| Never | 1,238 | 100.0 | 1229 | 99.3 | 9 | 0.7 | 1,227 | 99.1 | 11 | 0.9 | 1,170 | 94.5 | 68 | 5.5 | 1,233 | 99.6 | 5 | 0.4 | 1,227 | 99.1 | 11 | 0.9 | |||||||||||||||
| Ever | 546 | 100.0 | 546 | 100.0 | 0 | 0.0 | 0.037 | 544 | 99.6 | 2 | 0.4 | 0.189 | 528 | 96.7 | 18 | 3.3 | 0.027 | 546 | 100.0 | 0 | 0.0 | 0.161 | 543 | 99.5 | 3 | 0.5 | 0.336 | ||||||||||
| Drink | |||||||||||||||||||||||||||||||||||||
| Never | 1,478 | 100.0 | 1,469 | 99.4 | 9 | 0.6 | 1,465 | 99.1 | 13 | 0.9 | 1,404 | 95.0 | 74 | 5.0 | 1,473 | 99.7 | 5 | 0.3 | 1,465 | 99.1 | 13 | 0.9 | |||||||||||||||
| Ever | 306 | 100.0 | 306 | 100.0 | 0 | 0.0 | 0.183 | 306 | 100.0 | 0 | 0.0 | 0.086 | 294 | 96.1 | 12 | 3.9 | 0.260 | 306 | 100.0 | 0 | 0.0 | 0.390 | 305 | 99.7 | 1 | 0.3 | 0.279 | ||||||||||
| BMI (kg/m2) | |||||||||||||||||||||||||||||||||||||
| <18 | 59 | 100.0 | 58 | 98.3 | 1 | 1.7 | 56 | 94.9 | 3 | 5.1 | 55 | 93.2 | 4 | 6.8 | 59 | 100.0 | 0 | 0.0 | 58 | 98.3 | 1 | 1.7 | |||||||||||||||
| 18–24 | 900 | 100.0 | 895 | 99.4 | 5 | 0.6 | 893 | 99.2 | 7 | 0.8 | 859 | 95.4 | 41 | 4.6 | 898 | 99.8 | 2 | 0.2 | 893 | 99.2 | 7 | 0.8 | |||||||||||||||
| >24 | 825 | 100.0 | 822 | 99.6 | 3 | 0.4 | 0.361 | 822 | 99.6 | 3 | 0.4 | 0.000 | 784 | 95.0 | 41 | 5.0 | 0.715 | 822 | 99.6 | 3 | 0.4 | 0.787 | 819 | 99.3 | 6 | 0.7 | 0.718 | ||||||||||
| Protection | |||||||||||||||||||||||||||||||||||||
| Never | 307 | 100.0 | 304 | 99.0 | 3 | 1.0 | 305 | 99.3 | 2 | 0.7 | 287 | 93.5 | 20 | 6.5 | 307 | 100.0 | 0 | 0.0 | 305 | 99.3 | 2 | 0.7 | |||||||||||||||
| Ever | 1,477 | 100.0 | 1,471 | 99.6 | 6 | 0.4 | 0.191 | 1,466 | 99.3 | 11 | 0.7 | 0.607 | 1,411 | 95.5 | 66 | 4.5 | 0.088 | 1,472 | 99.7 | 5 | 0.3 | 0.307 | 1,465 | 99.2 | 12 | 0.8 | 0.557 | ||||||||||
| Education | |||||||||||||||||||||||||||||||||||||
| Junior high school | 48 | 100.0 | 48 | 100.0 | 0 | 0.0 | 47 | 97.9 | 1 | 2.1 | 43 | 89.6 | 5 | 10.4 | 48 | 100.0 | 0 | 0.0 | 46 | 95.8 | 2 | 4.2 | |||||||||||||||
| High school | 302 | 100.0 | 302 | 100.0 | 0 | 0.0 | 301 | 99.7 | 1 | 0.3 | 288 | 95.4 | 14 | 4.6 | 302 | 100.0 | 0 | 0.0 | 299 | 99.0 | 3 | 1.0 | |||||||||||||||
| College and above | 1,434 | 100.0 | 1,425 | 99.4 | 9 | 0.6 | 0.159 | 1,423 | 99.2 | 11 | 0.8 | 0.385 | 1,367 | 95.3 | 67 | 4.7 | 0.186 | 1,429 | 99.7 | 5 | 0.3 | 0.295 | 1,425 | 99.4 | 9 | 0.6 | 0.022 | ||||||||||
| Exposure time (years) | |||||||||||||||||||||||||||||||||||||
| <3 | 286 | 100.0 | 282 | 98.6 | 4 | 1.4 | 283 | 99.0 | 3 | 1.0 | 270 | 94.4 | 16 | 5.6 | 283 | 99.0 | 3 | 1.0 | 284 | 99.3 | 2 | 0.7 | |||||||||||||||
| ≥3 | 1,498 | 100.0 | 1,493 | 99.7 | 5 | 0.3 | 0.020 | 1,488 | 99.3 | 10 | 0.7 | 0.347 | 1,428 | 95.3 | 70 | 4.7 | 0.505 | 1,496 | 99.9 | 2 | 0.1 | 0.007 | 1,486 | 99.2 | 12 | 0.8 | 0.605 | ||||||||||
BMI, body mass index.
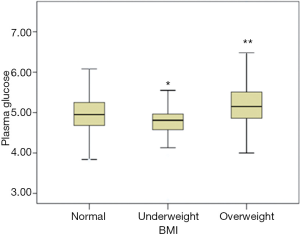
When considering three groups according to BMI, glucose level showed a significant increase in overweight (5.35±1.01) and decrease in underweight group (4.77±0.47) compared with normal group (5.05±0.85) (Figure 1). When taken all subjects as a whole, BMI was positively related with level of plasma glucose (r=0.20, P<0.01).
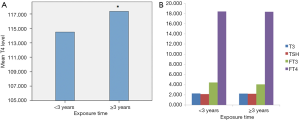
As Figure 2A,B has shown, we compared serum T3, T4, TSH, FT3 and FT4 levels between different degrees referred to exposure time of radiation. Subjects whose duration time longer than 3 years were likely to have higher T4 contents than those who contact less than 3 years (P<0.05). Unfortunately, no significant associations had been found between exposure time and serum T3, TSH, FT3 and FT4 levels.
Normal T4 subjects and abnormal ones were researched separately. In this study, serum T4 was significantly and positively related with BMI in the T4 normal group. Within normal T4 level, T4 was a little weak positive-correlated with BMI (r=0.06, P<0.01). For the T4 abnormal, relation between BMI and serum T4 were not statistical associated.
Discussion
We demonstrate a relevancy between thyroid function and gender in this study population, and with individuals grow older, the regulation function of thyroid related hormones appear to decline.
Another interesting result is the significant correlation between thyroid hormone levels and smoking status, subjects who never smoke may be susceptible to abnormal T3 and TSH compared to smokers. However, whether smoking is a protective factor against thyroid dysfunction needs to be considered carefully and scientifically.
The education degree influenced the thyroid hormones, with higher degree following lower abnormal incidence, implying the importance of knowledge and self-cultivation. When relating exposure time, subjects contacting X-ray longer than 3 years were unlikely to get abnormal, with the possible reason of physiologic compensatory reaction of organism, which will lead body to pathological symptom if they do not take remedial measures immediately after longer exposure.
Overall, there was a significant association of T4 level with exposure time when grouping the participants by radial exposure time and testing for hormone levels. These results indicate that thyroid function appears to change when workers are exposed to longer time of radial. However, this is not difficult to understand as higher radial levels are more detrimental, and a larger significant effect on thyroid would be expected if things continue on this way.
The present study has found an association between T4 and BMI. This relationship about them has been reported in some studies (17,18), considering that it may be mediated by leptin which is a type of protein produced by adipocytes, and adipocytes can regulate TRH secretion (19). It is well known that thyroid hormones associate with the maintenance of body weight (20). As we all known, hyperthyroidism is connected with weight loss and hypothyroidism is relative to a decreased metabolic rate (21,22), it is not a stretch to infer that abnormal weight is associated with hormonal changes, especially those related to thyroid function (23). It is well elucidated that the hypothalamic-pituitary-thyroid (HPT) axis participate in a large amount of metabolic processes, including energy expenditure and thermogenesis, affecting energy balance (24,25). Abnormal weight such as obesity may be the phenotypic expression of energy imbalance (26), accompanying with the results that abnormal weight may have altered HPT axis activity (27,28). Nevertheless, in abnormal weight status, whether determined changes of thyroid function are discussed controversially (29-32). However, measures of BMI were found to be positively related to T4 in our model, although in group of normal thyroid function.
Moreover, individuals whose weight being out of normal range are also the major patients suffered from metabolic syndrome deriving from insulin resistance, cardiovascular disease, and type 2 diabetes (33-36). In accordance with them, we found that BMI was closely related to plasma glucose level. As BMI increased, accompanying with glucose level arose, until pathological changes such as obesity and diabetes.
Although no causal association has been proved by these observational data, there are plausible medical explanations and other researches pointing in the same direction. Unfortunately, only few studies have reported the relevance between thyroid function and metabolic disorders so far. Since given that the increasing prevalence of abnormal weight, diabetes, and thyroid dysfunctions, further study in-depth the association between circulating thyroid function parameters and glucose-metabolic dysfunction are urgently needed in the future research.
Acknowledgments
We thank all subjects who participated in this study and Jiangsu Provincial Center for Disease Control and Prevention (JSCDC) that is our cooperating organization helping us recruiting participants in the study.
Funding: This study was supported by Jiangsu Provincial Medical Innovation Team (CXTDA2017029); Medical Science and Technology Development Foundation, Nanjing Department of Health (YKK14169) and Jiangsu Provincial Medical Youth Talent (QNRC2016127).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2017.03.07). XL serves as an unpaid Section Editor of Journal of Public Health and Emergency from Jun 2017 to Dec 2019. BZ serves as an Editor-in-Chief of Journal of Public Health and Emergency from Jan 2017 to Dec 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Regional Bioethical Committee at Jiangsu Provincial Center for Disease Control and Prevention (approval number: JSJK2015-B008-02) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abdel-Gayoum AA. Dyslipidemia and serum mineral profiles in patients with thyroid disorders. Saudi Med J 2014;35:1469-76. [PubMed]
- Mugii S, Hanada H, Okubo M, et al. Thyroid function influences serum apolipoprotein B-48 levels in patients with thyroid disease. J Atheroscler Thromb 2012;19:890-6. [Crossref] [PubMed]
- Vicinanza R, Coppotelli G, Malacrino C, et al. Oxidized low-density lipoproteins impair endothelial function by inhibiting non-genomic action of thyroid hormone-mediated nitric oxide production in human endothelial cells. Thyroid 2013;23:231-8. [Crossref] [PubMed]
- Mitchell JE, Hellkamp AS, Mark DB, et al. Thyroid function in heart failure and impact on mortality. JACC Heart Fail 2013;1:48-55. [Crossref] [PubMed]
- Singh S, Duggal J, Molnar J, et al. Impact of subclinical thyroid disorders on coronary heart disease, cardiovascular and all-cause mortality: a meta-analysis. Int J Cardiol 2008;125:41-8. [Crossref] [PubMed]
- Zamoiski RD, Cahoon EK, Freedman DM, et al. Prospective study of ultraviolet radiation exposure and thyroid cancer risk in the United States. Cancer Epidemiol Biomarkers Prev 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Wolny-Rokicka E, Tukiendorf A, Wydmanski J, et al. Thyroid Function after Postoperative Radiation Therapy in Patients with Breast Cancer. Asian Pac J Cancer Prev 2016;17:4577-81. [PubMed]
- Imaizumi M, Ohishi W, Nakashima E, et al. Association of radiation dose with prevalence of thyroid nodules among atomic bomb survivors exposed in childhood (2007-2011). JAMA Intern Med 2015;175:228-36. [Crossref] [PubMed]
- Ling S, Bhatt AD, Brown NV, et al. Correlative study of dose to thyroid and incidence of subsequent dysfunction after head and neck radiation. Head Neck 2017;39:548-54. [Crossref] [PubMed]
- Andersen SL, Olsen J, Laurberg P. Maternal thyroid disease in the Danish National Birth Cohort: prevalence and risk factors. Eur J Endocrinol 2016;174:203-12. [Crossref] [PubMed]
- Prinzi N, Sorrenti S, Baldini E, et al. Association of thyroid diseases with primary extra-thyroidal malignancies in women: results of a cross-sectional study of 6,386 patients. PLoS One 2015;10:e0122958 [Crossref] [PubMed]
- Fleiner HF, Bjoro T, Midthjell K, et al. Prevalence of Thyroid Dysfunction in Autoimmune and Type 2 Diabetes: The Population-Based HUNT Study in Norway. J Clin Endocrinol Metab 2016;101:669-77. [Crossref] [PubMed]
- Zou S, Wu F, Guo C, et al. Iodine nutrition and the prevalence of thyroid disease after salt iodization: a cross-sectional survey in Shanghai, a coastal area in China. PLoS One 2012;7:e40718 [Crossref] [PubMed]
- Herrera-Rangel AB, Aranda-Moreno C, Mantilla-Ochoa T, et al. Influence of the body mass index on the occurrence of falls in patients with type 2 diabetes mellitus. Obes Res Clin Pract 2015;9:522-6. [Crossref] [PubMed]
- Chikunguwo S, Brethauer S, Nirujogi V, et al. Influence of obesity and surgical weight loss on thyroid hormone levels. Surg Obes Relat Dis 2007;3:631-5; discussion 635-6. [Crossref] [PubMed]
- Fontenelle LC, Feitosa MM, Severo JS, et al. Thyroid Function in Human Obesity: Underlying Mechanisms. Horm Metab Res 2016;48:787-94. [Crossref] [PubMed]
- Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab 2005;90:4019-24. [Crossref] [PubMed]
- Michalaki MA, Vagenakis AG, Leonardou AS, et al. Thyroid function in humans with morbid obesity. Thyroid 2006;16:73-8. [Crossref] [PubMed]
- Popovic V, Duntas LH. Leptin TRH and ghrelin: influence on energy homeostasis at rest and during exercise. Horm Metab Res 2005;37:533-7. [Crossref] [PubMed]
- Wolters B, Lass N, Reinehr T. TSH and free triiodothyronine concentrations are associated with weight loss in a lifestyle intervention and weight regain afterwards in obese children. Eur J Endocrinol 2013;168:323-9. [Crossref] [PubMed]
- Seth B, Arora S, Singh R. Association of obesity with hormonal imbalance in infertility: a cross-sectional study in north Indian women. Indian J Clin Biochem 2013;28:342-7. [Crossref] [PubMed]
- Laurberg P, Knudsen N, Andersen S, et al. Thyroid function and obesity. Eur Thyroid J 2012;1:159-67. [Crossref] [PubMed]
- Chan DK, Tagamolila V, Ardhanari J, et al. Reference range of thyroid hormones in very low birth weight infants at the time of discharge. Thyroid 2014;24:73-7. [Crossref] [PubMed]
- Beck-Peccoz P, Mariotti S. Physiology of the Hypothalamic-Pituitary-Thyroid Axis. In: De Groot LJ, Chrousos G, Dungan K, et al. editors. SourceEndotext [Internet]. South Dartmouth (MA): MDText.com, Inc., 2000-. 2016 Aug 14.
- Z Zhou Y. The prevalence of impaired glucose regulation in anxiety disorder patients and the relationship with hypothalamic-pituitary-adrenal axis and hypothalamic-pituitary-thyroid axis activity. J Evid Based Med 2016; [Epub ahead of print].
- Benedetti FJ, Mocelin HT, Bosa VL, et al. Energy expenditure and estimated caloric intake in asthmatic adolescents with excess body weight. Nutrition 2010;26:952-7. [Crossref] [PubMed]
- Winchester S, Sullivan M. Diurnal patterns of hypothalamic-pituitary-adrenal axis function in preterm infants at young adulthood: Effects of prematurity, birth weight, and socioeconomic status. Psychoneuroendocrinology 2015;61:29-30. [Crossref] [PubMed]
- Grayson BE, Hakala-Finch AP, Kekulawala M, et al. Weight loss by calorie restriction versus bariatric surgery differentially regulates the hypothalamo-pituitary-adrenocortical axis in male rats. Stress 2014;17:484-93. [Crossref] [PubMed]
- Bjergved L, Jorgensen T, Perrild H, et al. Thyroid function and body weight: a community-based longitudinal study. PLoS One 2014;9:e93515 [Crossref] [PubMed]
- Lee JH, Kim SW, Jeon GW, et al. Thyroid dysfunction in very low birth weight preterm infants. Korean J Pediatr 2015;58:224-9. [Crossref] [PubMed]
- Koch L. Childhood weight gain increases risk of thyroid disorders. Nat Rev Endocrinol 2013;9:252. [Crossref] [PubMed]
- Soriguer F, Valdes S, Morcillo S, et al. Thyroid hormone levels predict the change in body weight: a prospective study. Eur J Clin Invest 2011;41:1202-9. [Crossref] [PubMed]
- Zivanovic D, Sipetic S, Stamenkovic-Radak M, et al. Potential risk factors for developing diabetes mellitus type 2. Med Pregl 2010;63:231-6. [Crossref] [PubMed]
- Maegawa H, Kashiwagi A. Obesity as risk for developing diabetes. Nihon Rinsho 2009;67:339-43. [PubMed]
- Seven E. Overweight, hypertension and cardiovascular disease: focus on adipocytokines, insulin, weight changes and natriuretic peptides. Dan Med J 2015;62:B5163. [PubMed]
- Horton ES, Silberman C, Davis KL, et al. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care 2010;33:1759-65. [Crossref] [PubMed]
Cite this article as: Li X, Zhu B, Tu L, Yu P, Song H, Liu X, Zhao L, Guo W, Zhao T, Liu J, Cao Y, Wang J, Yang D. Association between endocrine function and radiation exposure. J Public Health Emerg 2017;1:33.


