
Loss of 5-hydroxymethycytosine: enhancing precision in the diagnosis and prognosis of cutaneous malignant melanoma
The term ‘melanoma’ is derived from the Greek term ‘melas’, meaning ‘dark’, and ‘oma’, referring to tumor. Since its original, vivid, post-mortem 18th century descriptions, wherein “fatal black (tumors) with metastases and black fluid in the body” were observed at autopsy, melanoma remains one of the most virulent of all human malignancies and a persistent threat to public health (1). This deadly form of cancer results from the malignant transformation of the melanocyte, the pigment-producing cells present in the skin and other organ systems within the human body. While those with blonde or red hair and blue eyes are known to be at greatest risk, all populations, including those of Asian and African descent, are afflicted (2,3). An estimated 76,380 new cases of invasive melanoma will be diagnosed in the United States in 2016 (4). In addition, while it accounts for less than one percent of all skin cancer cases, melanoma is responsible for the majority of skin cancer deaths (4). Upon reaching a diagnosis of melanoma, its depth of invasion as determined by simple micrometer measurement (Breslow depth) has been the gold standard of prognosis for the past 40 years. Prognostication of primary cutaneous melanoma is based on this key histologic feature and forms the basis upon which further diagnostic and treatment strategies are determined (5). Other microscopic features, such as ulceration and mitosis, were included in the 2009 edition of the American Joint Committee on Cancer (AJCC) staging guidelines. However, recent data indicate that mitosis is not an accurate prognostic parameter in larger cohorts and has, thus, been removed from the updated 2017 AJCC staging guidelines for melanoma. In reality, however, clinical experience has shown that outcomes can vary significantly within a given AJCC stage, indicating the need for improvements with this model. Such inaccuracies in melanoma prognostication could ultimately lead to over- or under-treatment, either of which can be consequential. International efforts are currently underway to revise and update the AJCC melanoma staging system.
Of relevance, Saldanha and colleagues [2017] recently investigated the potential diagnostic and prognostic utility of an emerging, adjunctive diagnostic epigenetic biomarker, 5-hydroxymethylcytosine (5-hmC), in cutaneous melanoma (6). Their analysis of 5-hmC immunohistochemical expression in 200 clinically-annotated cases of primary cutaneous melanomas of varying stages with an average of greater than 6 years follow-up represents the largest cohort to date to be studied with this putative biomarker. Multivariate analysis of this cohort demonstrated that 5-hmC immunoreactivity was significantly associated with metastasis-free survival, melanoma-specific survival, and overall survival wherein those with the lowest levels of 5-hmC expression had the worst outcomes. In addition, these findings remained true even after adjusting for confounding variables, strongly suggesting that this epigenetic marker could serve as an independent predictor of prognosis. Moreover, the addition of 5-hmC to a proportional hazards model that compared initial tumor AJCC stage and clinical outcome improved the overall fit of the model, which led the authors to propose use of this marker to enhance precision of the existing AJCC melanoma staging schema.
5-hydroxymethylcytosine (5-hmC) is an oxidized form of 5-methylcytosine and was recently demonstrated to serve as a key biochemical intermediate of the DNA de-methylation pathway (7) (Figure 1A). This recently described, fundamental biological mechanism enables the removal of methyl groups off of the DNA base cytosine and is catalyzed by a family of dioxygenase enzymes called Ten-Eleven Translocase (TET) (7). In animal models, melanomas lacking this enzyme yielded more aggressive, lethal tumors compared to those that maintained this key enzyme (9). Recent data indicate that loss of function of this putative tumor suppressor through direct genetic mutation, oncometabolite accumulation, or epigenetic silencing may lead to its pathogenic dysfunction (10,11). Emerging data also suggest that dysfunction to this pathway may represent an early, important pathogenic event in the malignant transformation of melanocytes (12). Based on these seminal observations, studies are ongoing to investigate the translational therapeutic potential of these findings. Importantly, loss of function of this enzyme, as inferred by 5-hmC loss, is not unique to melanoma and has been demonstrated in human malignancies of other organ systems (Table 1) (8,13-16).
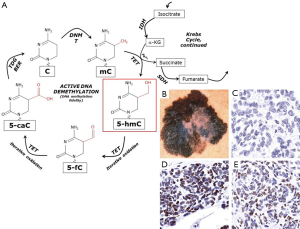
Table 1
| Loss of 5-hydroxymethylcytosine | Reference |
|---|---|
| Oromucosal squamous cell carcinoma | Jäwert et al. 2013 (13) |
| Gastric carcinoma | Yang et al. 2013 (14) |
| Pancreatic carcinoma | Bhattacharyya et al. 2013 (15) |
| Prostate carcinoma | Haffner et al. 2011 (16) |
| Breast carcinoma | Haffner et al. 2011 (16) |
| Colorectal carcinoma | Haffner et al. 2011 (16) |
Equally important to the potential utility of this marker in enhancing precision in staging of primary cutaneous melanoma is its applicability to a number of challenging diagnostic scenarios. One such example is in the pathologic evaluation of sentinel lymph node biopsies, which are traditionally offered as a staging procedure to those patients with no clinical evidence of metastatic disease and a primary melanoma that invades deeper than one millimeter or is thinner but has other “high risk features”, such as evidence of mitotic activity, ulceration, or lymphovascular invasion. During the sentinel lymph node evaluation, a benign collection of melanocytes, referred to as a nodal nevus, is not infrequently encountered and is a relatively common, benign mimicker of metastatic melanoma (17). An accurate assessment of sentinel lymph node status is not trivial, as those with evidence of lymph node metastases have a significantly worse prognosis and are recommended additional invasive diagnostic procedures and treatments that can be associated with significant morbidity. To this end, we have recently shown that 5-hmC, which is strongly expressed in benign cutaneous nevi but strikingly lost in primary cutaneous melanoma, can help distinguish nodal nevi from metastatic melanoma with high sensitivity and specificity (9,18). In addition, a very recent report demonstrated that detection of 5-hmC by immunohistochemistry can discriminate melanomas from benign proliferative nodules arising within giant congenital nevi (19). Thus, evidence is accumulating to support that 5-hmC is indeed a reliable adjunctive biomarker help reach the correct diagnosis in ambiguous scenarios when histological and routine immunohistochemical characteristics are insufficient or non-specific.
Analysis of 5-hmC content by immunohistochemistry may also be useful in the assessment of melanocytic tumors that evade definitive histologic diagnosis. A subset of melanocytic tumors do not fit criteria sufficient for a diagnosis of malignant melanoma but bear features that exclude them from well-established categories of benign but ‘atypical’ melanocytic neoplasms, such as the dysplastic nevus (20). The nomenclature of these indeterminate neoplasms is varied and inconsistent in the literature. Experts in the field have recommended that such a melanocytic neoplasm be referred to as a melanocytic tumor of uncertain malignant potential (MELTUMP) (20). Recently, we have observed that semi-quantification of 5-hmC immunohistochemical expression can help stratify such cases of MELTUMP and potentially identify those with a worse prognosis and, thereby, guide appropriate treatment (Figure 1B,C,D).
In summary, 5-hmC is an emerging diagnostic biomarker with significant translational potential for enhancing precision of melanoma staging, distinguishing benign from malignant melanocytic neoplasms, and aiding in the pathologic assessment of those that fall somewhere in between. Saldanha and colleagues’ important observations provide strong support for the continued development and maturation of this biomarker to improve accuracy and precision of melanoma AJCC staging guidelines. As melanoma is one of the most virulent forms of human malignancy, continued international efforts to explore the diagnostic and therapeutic applications of 5-hmC and TET enzyme dysregulation in melanoma are warranted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Public Health and Emergency. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jphe.2017.02.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Urteaga O, Pack GT. On the antiquity of melanoma. Cancer 1966;19:607-10. [Crossref] [PubMed]
- Robles-Espinoza CD, Roberts ND, Chen S, et al. Germline MC1R status influences somatic mutation burden in melanoma. Nat Commun 2016;7:12064. [Crossref] [PubMed]
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol 2009;145:427-34. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society, 2016.
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206. [Crossref] [PubMed]
- Saldanha G, Joshi K, Lawes K, et al. 5-Hydroxymethylcytosine is an independent predictor of survival in malignant melanoma. Mod Pathol 2017;30:60-8. [Crossref] [PubMed]
- Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009;324:930-5. [Crossref] [PubMed]
- Lee JJ, Murphy GF, Lian CG. Melanoma epigenetics: novel mechanisms, markers, and medicines. Lab Invest 2014;94:822-38. [Crossref] [PubMed]
- Lian CG, Xu Y, Ceol C, et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 2012;150:1135-46. [Crossref] [PubMed]
- Song F, Amos CI, Lee JE, et al. Identification of a melanoma susceptibility locus and somatic mutation in TET2. Carcinogenesis 2014;35:2097-101. [Crossref] [PubMed]
- Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011;19:17-30. [Crossref] [PubMed]
- Lian CG, Murphy GF. The Genetic Evolution of Melanoma. N Engl J Med 2016;374:994-5. [PubMed]
- Jäwert F, Hasseus B, Kjeller G, et al. Loss of 5-hydroxymethylcytosine and TET2 in oral squamous cell carcinoma. Anticancer Res 2013;33:4325-8. [PubMed]
- Yang Q, Wu K, Ji M, et al. Decreased 5-hydroxymethylcytosine (5-hmC) is an independent poor prognostic factor in gastric cancer patients. J Biomed Nanotechnol 2013;9:1607-16. [Crossref] [PubMed]
- Bhattacharyya S, Yu Y, Suzuki M, et al. Genome-wide hydroxymethylation tested using the HELP-GT assay shows redistribution in cancer. Nucleic Acids Res 2013;41:e157 [Crossref] [PubMed]
- Haffner MC, Chaux A, Meeker AK, et al. Global 5-hydroxymethylcytosine content is significantly reduced in tissue stem/progenitor cell compartments and in human cancers. Oncotarget 2011;2:627-37. [Crossref] [PubMed]
- Carson KF, Wen DR, Li PX, et al. Nodal nevi and cutaneous melanomas. Am J Surg Pathol 1996;20:834-40. [Crossref] [PubMed]
- Lee JJ, Granter SR, Laga AC, et al. 5-Hydroxymethylcytosine expression in metastatic melanoma versus nodal nevus in sentinel lymph node biopsies. Mod Pathol 2015;28:218-29. [Crossref] [PubMed]
- Pavlova O, Fraitag S, Hohl D. 5-Hydroxymethylcytosine Expression in Proliferative Nodules Arising within Congenital Nevi Allows Differentiation from Malignant Melanoma. J Investig Dermatol 2016;136:2453-61. [Crossref] [PubMed]
- Cerroni L, Barnhill R, Elder D, et al. Melanocytic tumors of uncertain malignant potential: results of a tutorial held at the XXIX Symposium of the International Society of Dermatopathology in Graz, October 2008. Am J Surg Pathol 2010;34:314-26. [Crossref] [PubMed]
Cite this article as: Lee JJ, Lian CG. Loss of 5-hydroxymethycytosine: enhancing precision in the diagnosis and prognosis of cutaneous malignant melanoma. J Public Health Emerg 2017;1:24.


